Preparation method of mesotrione
A technology for mesotrione and nitrobenzoic acid is applied in the field of preparation of mesotrione, can solve problems such as potential safety hazards, environmental pollution, low reaction activity, etc., achieves small amount of three wastes, reduced production cost, and low reaction temperature Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
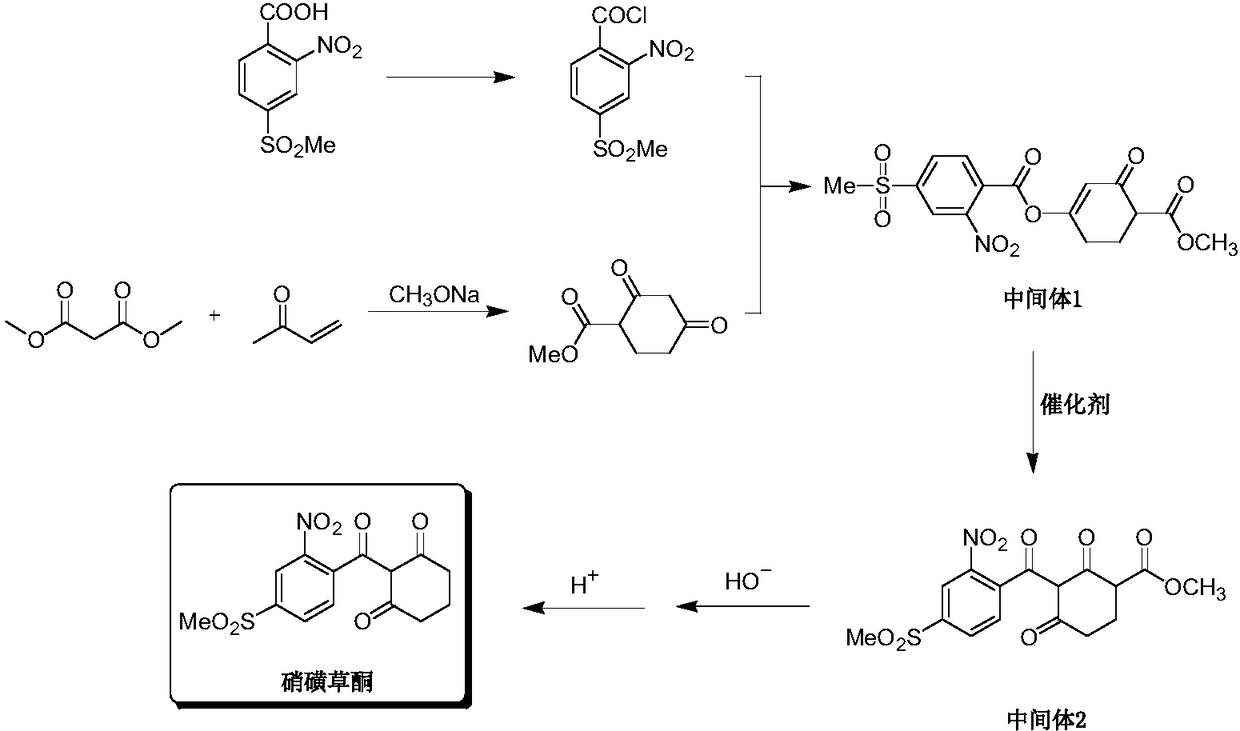
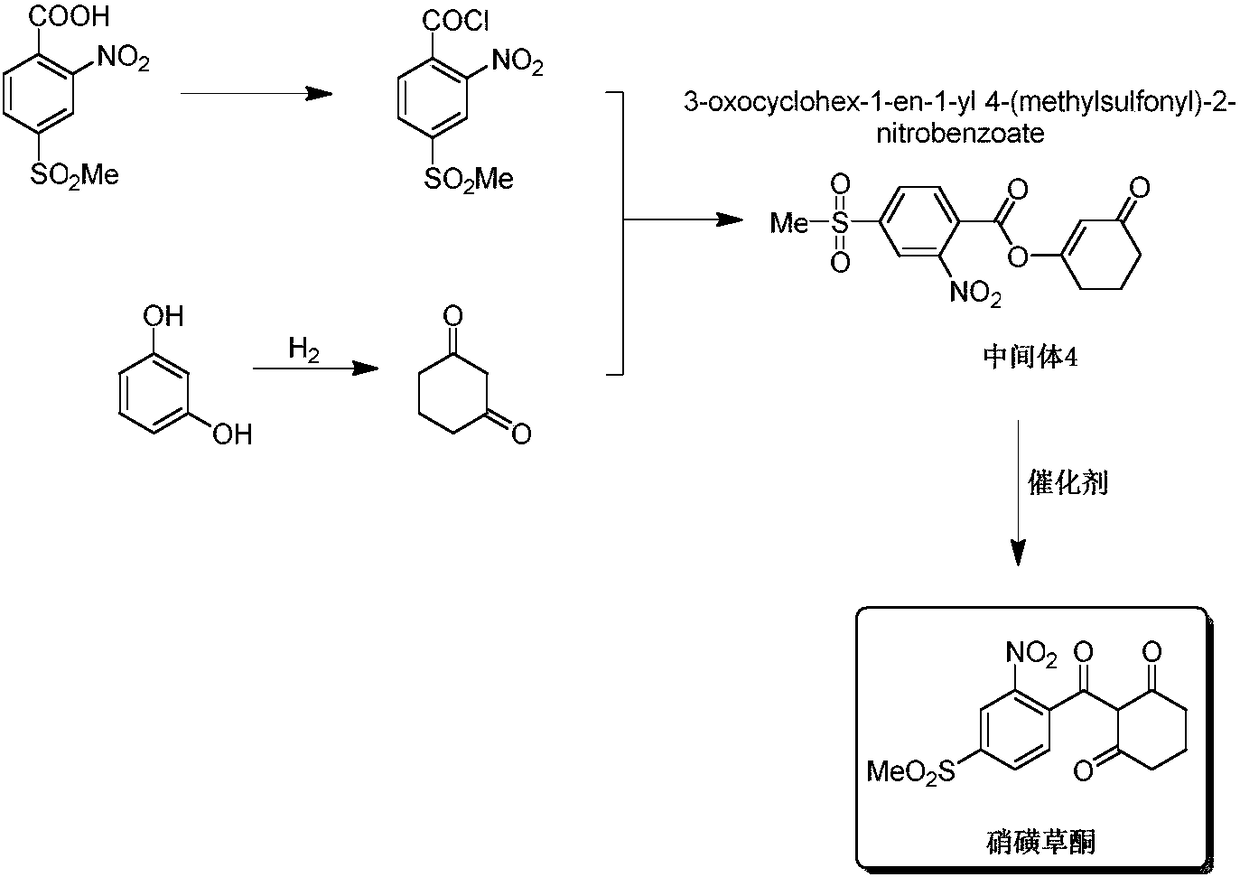
[0045] Drop into the dry reaction flask 25g (0.101mol) of p-thiamone-o-nitrobenzoic acid and 75g of ethylene dichloride, stir and heat up to reflux, add 13g (0.108mol) of thionyl chloride dropwise, and return to reflux after dropping. The temperature was reacted for 2 hours, and the solvent was distilled off under reduced pressure to obtain 26.6 g of p-thiamone-o-nitrobenzoyl chloride.
[0046] Add 80g of acetonitrile to the above-obtained p-methylsulfone-o-nitrobenzoyl chloride, add 12g (0.106mol) of 1,3-cyclohexanedione at 0-30°C, and add triethyl ether dropwise at 0-30°C 11g (0.108mol) of amine, dripping in 1-2 hours, and kept at 20-30°C for 2 hours to obtain the intermediate 2-nitro-4-methylsulfonylbenzoic acid-[3'-carbonyl-1'-cyclohexyl enol]-ester.
[0047] Add 9.8 g (0.03 mol) of cesium carbonate and 0.5 g (0.004 mol) of 4-dimethylaminopyridine into the reaction system, keep warm at 20-30° C. for 3 hours, evaporate the solvent under reduced pressure, add 30 g of water,...
Embodiment 2
[0049] Add 25g (0.101mol) of p-thiamone-o-nitrobenzoic acid and 125g of ethylene dichloride into the dry reaction flask, stir and heat up to reflux, add 17.85g (0.15mol) of thionyl chloride dropwise, and dropwise the reflux temperature After reacting for 1 hour, the solvent was distilled off under reduced pressure to obtain 26 g of p-thiamphenyl-o-nitrobenzoyl chloride.
[0050]Add 125 g of dichloroethane to the above-obtained p-methylsulfone-o-nitrobenzoyl chloride, add 12 g (0.106 mol) of 1,3-cyclohexanedione at 20-30 ° C, and dropwise add three Ethylamine 22g (0.2mol), dripped in 2 hours, and kept at 20-30°C for 1 hour to obtain the intermediate 2-nitro-4-methylsulfonylbenzoic acid-[3'-carbonyl-1'-cyclohexene alcohol]-ester.
[0051] Add 17g (0.05mol) of cesium carbonate (0.05mol) and 0.5g (0.003mol) of DBU to the reaction system, keep the temperature at 20-30°C for 2 hours, evaporate the solvent under reduced pressure, add 30g of water, acidify with hydrochloric acid, Ex...
Embodiment 3
[0053] Add 100 g of dichloroethane to the p-thiamone-o-nitrobenzoyl chloride obtained according to the method in Example 1, add 12 g (0.106 mol) of 1,3-cyclohexanedione at 20-30 ° C, and 11g (0.108mol) of triethylamine was added dropwise at ℃, the drop was completed in 1-2 hours, and the temperature was kept at 20-30℃ for 2 hours to obtain the intermediate 2-nitro-4-methylsulfonylbenzoic acid-[3'-carbonyl- 1'-cyclohexenol]-ester.
[0054] Add 15g (0.1mol) of potassium carbonate and 0.15g (0.001mol) of hexamethylenetetramine into the reaction system, keep warm at 20-30°C for 6 hours, add 30g of water, acidify with hydrochloric acid, extract, dry, and reduce pressure The solvent was distilled off to obtain 29.7 g (0.082 mol) of the product mesotrione, with a content of 97% and a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com