Multifunctional imidazolium surfactant and preparation method thereof
A surfactant and multi-functionality technology, applied in chemical instruments and methods, organic chemistry, dissolution, etc., can solve problems such as difficulty in forming densely arranged structures, large electrostatic repulsion, and increased usage, and achieve easy separation, purification, and enhancement Hydrophilicity and the effect of less by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
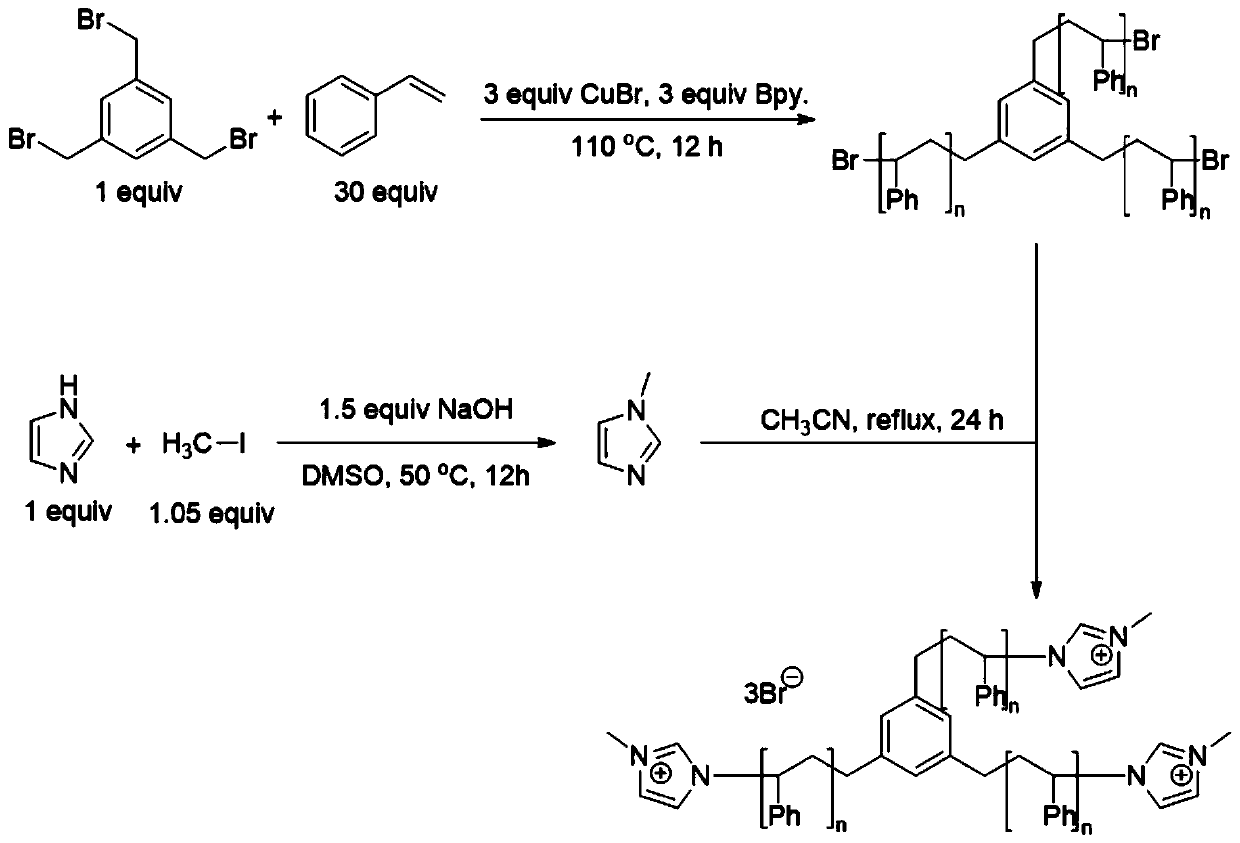
[0026] Step 1. Dissolve 0.68g imidazole (10mmol) in dimethyl sulfoxide (20mL), heat to 50°C, add 0.6g sodium hydroxide (15mmol) under stirring, continue stirring for 15min, then add 1.49g methyl iodide dropwise (10.5mmol), after the dropwise addition is completed, continue to react for 12h, cool to room temperature, dilute with water, extract 3 times with dichloromethane, combine the organic phases, wash 3 times with water, add water-removing agent anhydrous sulfuric acid in the organic phase Sodium, after standing still for 1h, suction filtration, after removing the solvent, N-methylimidazole was obtained with a yield of 98%;
[0027] Step 2, under the protection of nitrogen, add 3.12g styrene (30mmol) deoxygenated in advance in the reaction flask, then add 0.357g s-tris(bromomethyl)benzene (1mmol), 0.432g cuprous bromide (3mmol) successively ) and 0.468g of bipyridine (3mmol), stirred at room temperature for 10min, heated to 110°C, stirred for 12h, cooled to room temperature...
Embodiment 2
[0031] Step 1. Dissolve 0.82g 2-methylimidazole (10mmol) in N,N-dimethylformamide (20mL), heat to 40°C, add 0.84g potassium hydroxide (15mmol) while stirring, and continue stirring After 15 minutes, add 1.438 g of bromobutane (10.5 mmol) dropwise. After the dropwise addition, continue the reaction for 12 hours, cool to room temperature, dilute with water, extract 3 times with dichloromethane, combine the organic phases, and wash 3 times with water , adding a dehydrating agent, anhydrous sodium sulfate, to the organic phase, and after standing still for 1 hour, suction filtration, after removing the solvent, 1-butyl-2-methylimidazole was obtained with a yield of 97%;
[0032] Step 2, under the protection of nitrogen, add 1.77g of α-methylstyrene (15mmol) deoxygenated in advance to the reaction flask, and then add 0.357g of s-tris(bromomethyl)benzene (1mmol), 0.432g of ethylene bromide Copper (3mmol) and 0.468g bipyridine (3mmol), stirred at room temperature for 10min, heated to...
Embodiment 3
[0036] Step 1. Dissolve 1.442g 2-phenylimidazole (10mmol) in N,N-dimethylacetamide (20mL), control the temperature at 20°C, add 0.36g sodium hydride (15mmol) under stirring, and continue stirring After 15min, add 1.144g bromoethane (10.5mmol) dropwise. After the dropwise addition, continue the reaction for 12h, cool to room temperature, dilute with water, extract 3 times with dichloromethane, combine the organic phases, wash 3 times with water, and A dehydrating agent, anhydrous sodium sulfate, was added to the organic phase, and after standing still for 1 hour, it was suction filtered, and after removing the solvent, 1-ethyl-2-phenylimidazole was obtained with a yield of 98%;
[0037]Step 2, under the protection of nitrogen, add 2.1g methyl methacrylate (21mmol) deoxygenated in advance to the reaction flask, then add 0.357g s-tris(bromomethyl)benzene (1mmol), 0.432g ethylene bromide successively Copper (3mmol) and 0.468g bipyridine (3mmol), stirred at room temperature for 10m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com