Synthesizing method of linear polyethyleneimine block copolymer
A polyethyleneimine block and synthesis method technology, applied in the field of polymer materials, can solve the problems that nitrogen heterocyclic carbene is not suitable for industrial scale-up and limits the application of polyethyleneimine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
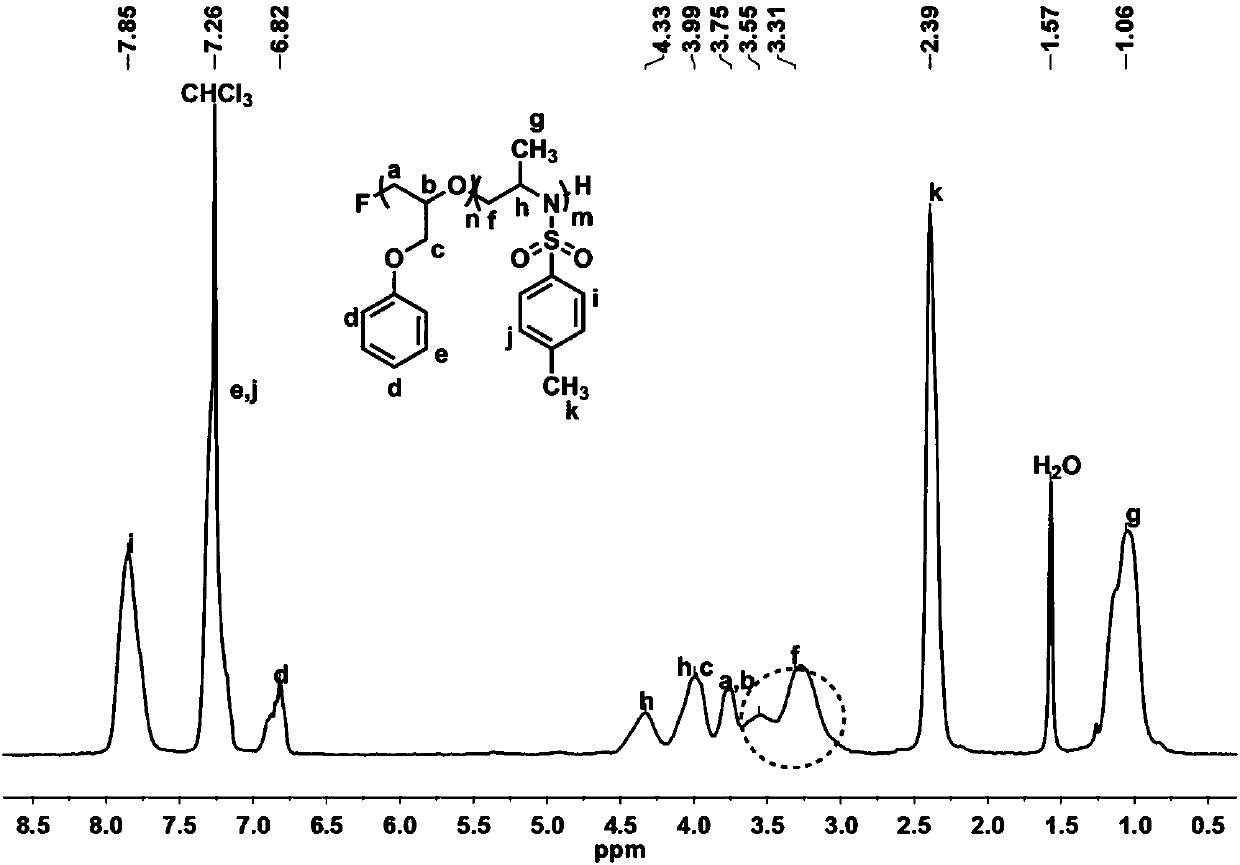
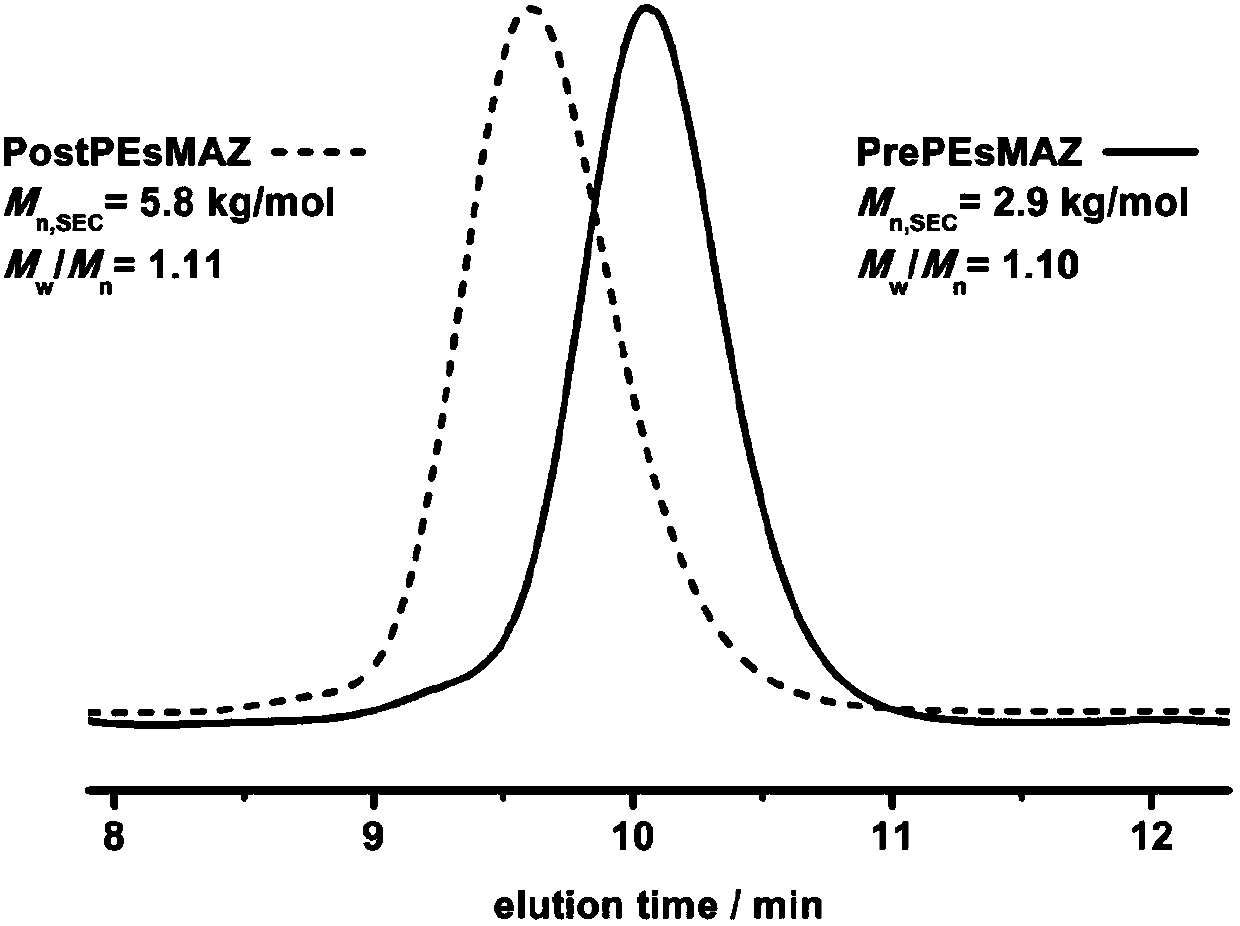
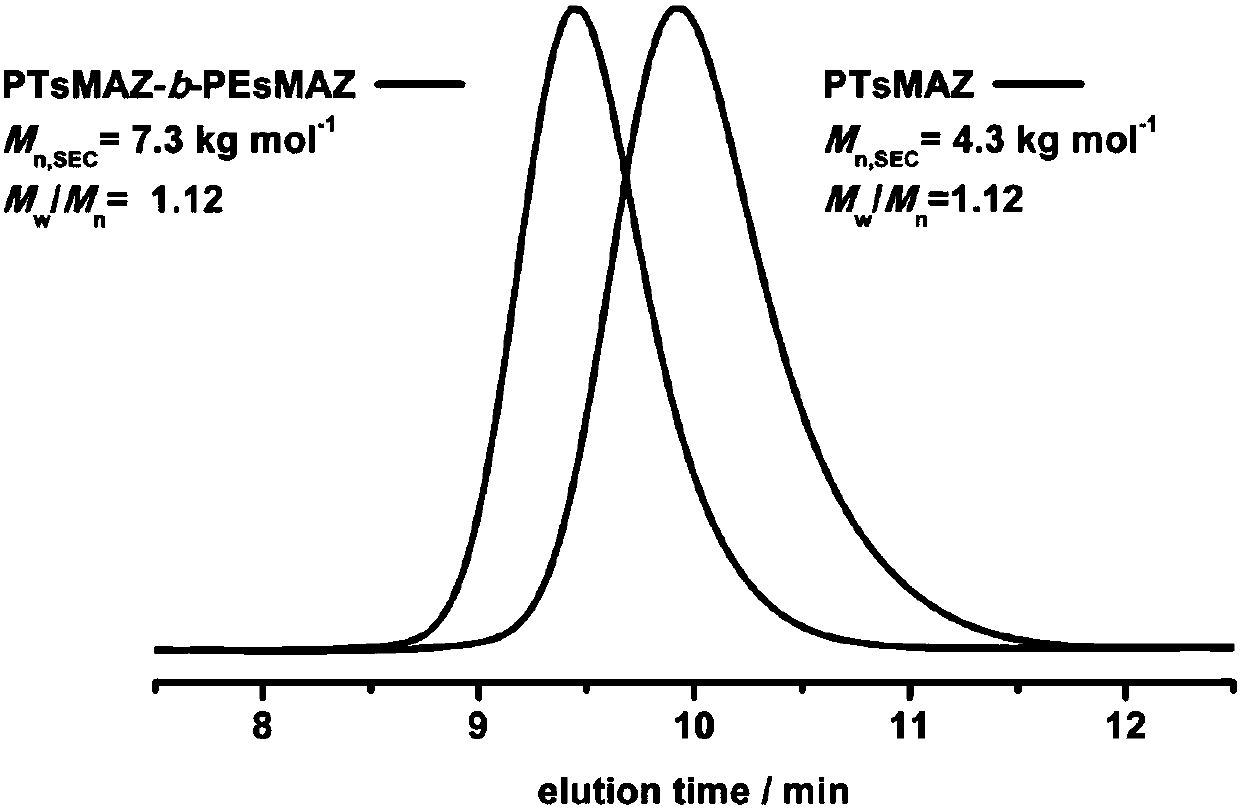
[0096] Under nitrogen flow purging, add THF solution (0.06mmol, lequiv) of tetrabutylammonium fluoride to the reaction bottle equipped with magnetic stirring bar, add phenylglycidyl ether (Z) after removing THF under reduced pressure (1.2 mmol, 20 equiv). Then the system was placed in an oil bath at 50° C. for 3-4 hours, and the consumption of GPE was monitored by 1H NMR. The monomers were completely consumed and did not stir. Add dimethyl sulfoxide (1 mL) to the system to dissolve the system, and wait until it is completely dissolved. 2-Methyl-1-(p-tolylsulfonyl)aziridine (H) (1.2 mmol, 20 equiv) was added to continue the reaction for 2 h. Finally, the reaction solution was dropped into excess methanol to precipitate a white polymer. After removing the upper layer of methanol by centrifugation, the crude product was washed with cold methanol for 3-4 times to obtain a pure product. After drying it in a vacuum oven for 2 days, it was analyzed by hydrogen spectrometry. The co...
Embodiment 2
[0098]Under nitrogen flow purging, add THF solution (0.06mmol, lequiv) of tetrabutylammonium chloride to the reaction bottle equipped with magnetic stirring bar, add phenylglycidyl ether (Z) after removing THF under reduced pressure (1.8 mmol, 30 equiv). Then the system was placed in an oil bath at 50° C. for 3-4 hours, and the consumption of GPE was monitored by 1H NMR. The monomers were completely consumed and did not stir. Add dimethyl sulfoxide (1 mL) to the system to dissolve the system, and wait until it is completely dissolved. 2-Methyl-1-(p-tolylsulfonyl)aziridine (H) (1.8 mmol, 30 equiv) was added to continue the reaction for 2 h. Finally, the reaction solution was dropped into excess methanol to precipitate a white polymer. After removing the upper layer of methanol by centrifugation, the crude product was washed with cold methanol for 3-4 times to obtain a pure product. After drying it in a vacuum oven for 2 days, it was analyzed by hydrogen spectrometry. The con...
Embodiment 3
[0100] Under nitrogen flow purging, add THF solution (0.06mmol, lequiv) of tetrabutylammonium bromide to the reaction flask equipped with magnetic stirring bar, add phenyl glycidyl ether (Z) after removing THF under reduced pressure (1.8 mmol, 30 equiv). Then the system was placed in an oil bath at 50° C. for 3-4 hours, and the consumption of GPE was monitored by 1H NMR. The monomers were completely consumed and did not stir. Add dimethyl sulfoxide (1 mL) to the system to dissolve the system, and wait until it is completely dissolved. 2-Methyl-1-(p-tolylsulfonyl)aziridine (H) (1.8 mmol, 30 equiv) was added to continue the reaction for 2 h. Finally, the reaction solution was dropped into excess methanol to precipitate a white polymer. After removing the upper layer of methanol by centrifugation, the crude product was washed with cold methanol for 3-4 times to obtain a pure product. After drying it in a vacuum oven for 2 days, it was analyzed by hydrogen spectrometry. The con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com