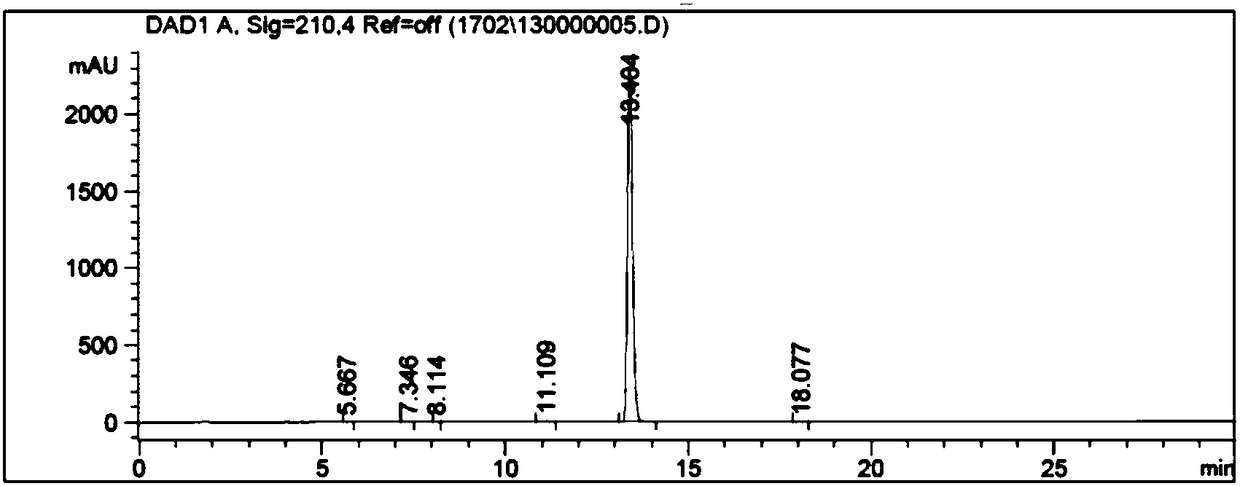
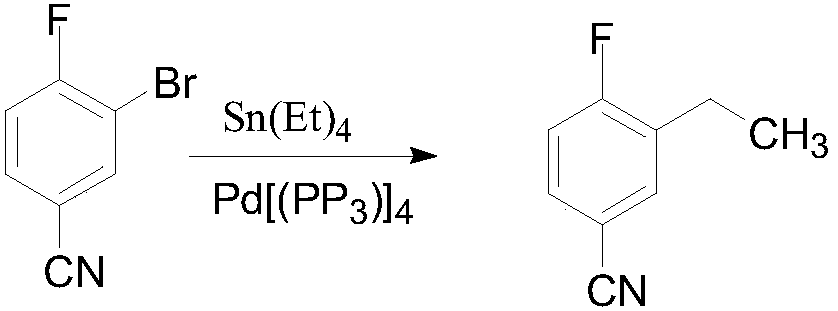
3-ethyl-4-fluorobenzonitrile preparation method
A technology of fluorobenzonitrile and ethyl, which is applied in the field of preparation of 3-ethyl-4-fluorobenzonitrile, can solve problems such as difficult industrial production, hazards to operators and the environment, and no description of the specific purity of the product.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 3-bromo-4-fluorobenzonitrile
[0044]
[0045] Dissolve 3-bromo-4-fluorobenzaldehyde (250g, 1.23mol) in acetonitrile (1.5L), then add sulfamic acid (67g, 1.48mol), and reflux for 4h. TLC shows that the conversion of the raw material is complete, and the reaction solution is concentrated To a small volume, add water (2L) and stir for 30 minutes, cool to 5-10°C and continue stirring for 10 minutes, filter, dissolve the filter cake with methyl tert-butyl ether (1.2L), wash twice with 500ml water, and saturate with 200ml Wash once with sodium bicarbonate solution, dry over anhydrous sodium sulfate, filter, absorb the filtrate with activated carbon (10g), filter, concentrate under reduced pressure to remove the solvent, add n-heptane (250ml), cool and stir in an ice-salt bath for 1h, filter, reduce Drying under pressure gave 3-bromo-4-fluorobenzonitrile (217 g, yield 88%). 1 H NMR (CDCl 3 ,400MHz): δ7.91(m,1H),7.63(m,1H),7.24(m,1H).
Embodiment 2
[0046] Example 2 3-bromo-4-fluorobenzonitrile
[0047]
[0048] Add tetrahydrofuran (100ml) into a 250ml reaction flask, add 3-bromo-4-fluorobenzaldehyde (10g, 49.2mmol) and ammonia water (40ml) under stirring, add elemental iodine (25g, 98.5mmol) in batches under cooling to 5°C ), then rose to ambient temperature and reacted for 2 to 3 hours. After the reaction was completed, the reaction solution was poured into 10% aqueous solution of sodium sulfite (200g), extracted twice with methyl tert-butyl ether (100ml), and dried over anhydrous sodium sulfate. , concentrated under reduced pressure to remove the solvent, added n-heptane (20ml), cooled to 0-10°C and stirred for 1h, filtered, and dried under reduced pressure to give 3-bromo-4-fluorobenzonitrile (9.6g, yield: 97.5 %). The NMR spectrum of this compound was determined to be the same as the product of Example 1.
Embodiment 3
[0049] Example 3 3-Ethyl-4-fluorobenzonitrile
[0050]
[0051]3-Bromo-4-fluorobenzonitrile (200g, 1mol) and [1,1-bis(diphenylphosphino)ferrocene]palladium(II) chloride dichloromethane complex (4.08g, 5mmol) was dissolved in THF (1.2L), and 1.0M / L diethylzinc-n-hexane solution (600mL, 0.6mol) was added at 40-50°C. After dripping, the temperature was raised to 50-60°C for 4-5h. The raw material reacted completely. After the reaction solution was cooled to room temperature, it was added to 5% dilute hydrochloric acid (1 L), and the layers were separated. The organic layer was washed twice with 500 ml of water, and then concentrated under reduced pressure to remove the solvent. Then add n-hexane (600mL) and activated carbon (20g), reflux for 0.5h, cool to room temperature, filter, then add activated carbon (10g) to the filtrate, reflux for 0.5h, cool to room temperature, filter, and cool to -50°C to -60°C and filtered, and the filter cake was dried under reduced pressure at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com