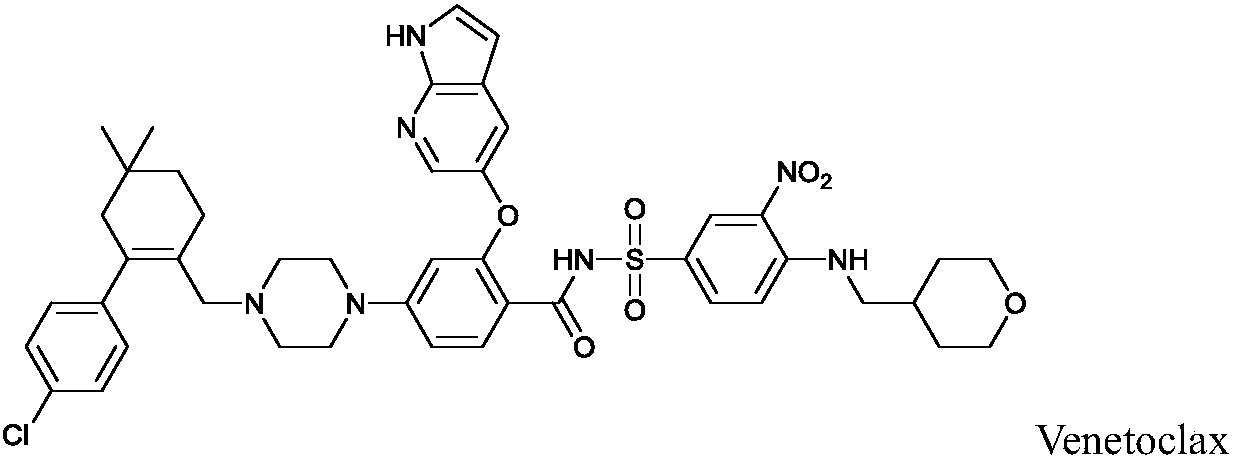
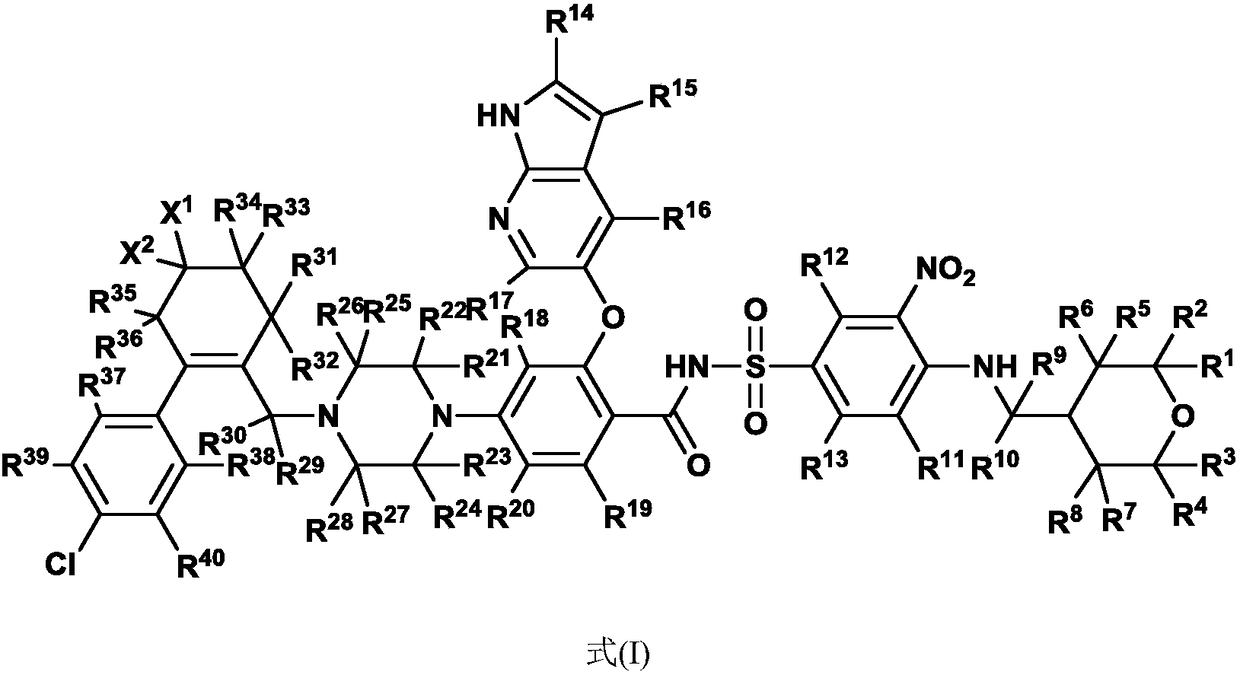
N-benzenesulfonyl benzamide compound for inhibiting Bcl-2 proteins as well as composition and application thereof
A technology of benzenesulfonylbenzamide and bcl-2, applied in the field of medicine, can solve problems such as poor patient compliance, poor absorption, distribution, metabolism and/or excretion, and limited application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085] The preparation of the compounds of the present invention may involve the protection and deprotection of various chemical groups. The need for protection and deprotection and selection of appropriate protecting groups can be readily determined by those skilled in the art. The chemistry of protecting groups can be found in, eg, Wuts and Greene, Protective Groups in Organic Synthesis, 4th Edition, John Wiley & Sons: New Jersey, (2006), which is incorporated herein by reference in its entirety.
[0086] The reaction can be monitored according to any suitable method known in the art. For example, spectroscopic means such as nuclear magnetic resonance (NMR) spectroscopy (e.g. 1 H or 13 C), infrared (IR) spectroscopy, spectrophotometry (e.g., UV-visible), mass spectroscopy (MS)) or by chromatographic methods such as high performance liquid chromatography (HPLC) or thin layer chromatography (TLC) Product formation was monitored.
[0087] Pharmaceutical compositions, prep...
Embodiment 1
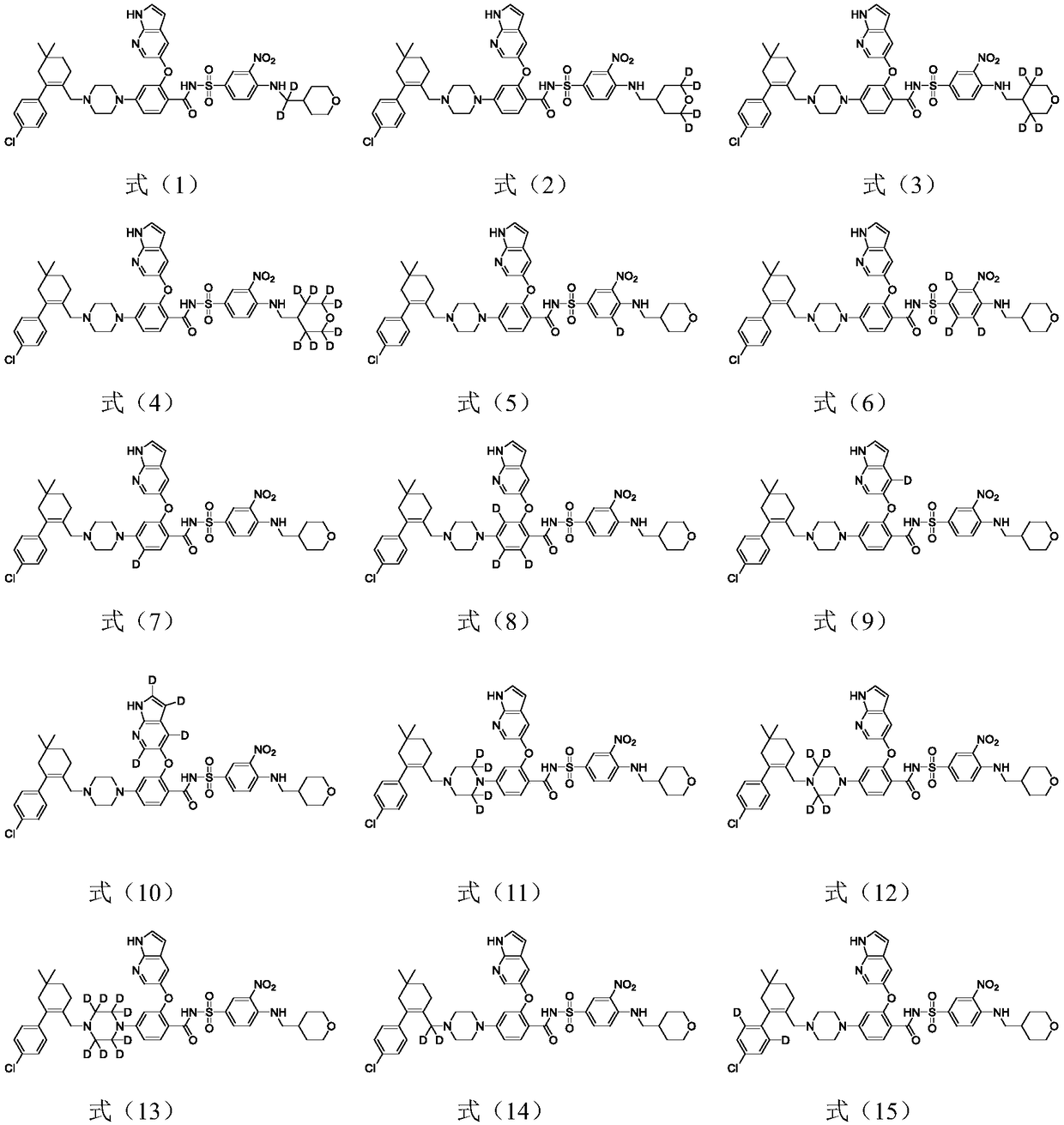
[0119] Example 1 Preparation of 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl-d 2}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl)amino]phenyl}sulfonyl)-2-(1H- Pyrrolo[2,3-b]pyridin-5-yloxy)benzamide, namely compound T-1, has the following molecular formula:
[0120]
[0121] Synthesize using the following route:
[0122]
[0123] Step 1 Synthesis of Compound 5.
[0124] Under nitrogen protection, sodium hydride (1.9g, 79.3mmol) and dimethyl carbonate (14.3g, 158.6mmol) were added to anhydrous THF (15ml) solution and heated, and 3,3-dimethylcyclohexane was added dropwise at reflux The THF solution of the ketone (5.0 g, 39.6 mmol) continued to reflux for 4 h after the dropwise addition was completed. Cool to room temperature, add methanol to quench the reaction, then add water and dichloromethane for extraction, collect the organic phase, and separate and purify by column chromatography to obtain 4.2 g of a colorless liquid product with...
Embodiment 2
[0143] Example 2 Preparation of 4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-en-1-yl]methyl-d 2}piperazin-1-yl)-N-({3-nitro-4-[(tetrahydro-2H-pyran-4-ylmethyl-d 2 )amino]phenyl}sulfonyl)-2-(1H-pyrrolo[2,3-b]pyridin-5-yloxy)benzamide, namely compound T-2, the molecular formula is as follows:
[0144]
[0145] Adopt the following synthetic route:
[0146]
[0147] Step 1 Synthesis of compound 15.
[0148] At 0°C, the LiAlD 4 (0.4g, 10.08mmol) was slowly added dropwise to a solution of compound 14 (1.0g, 9.00mmol) in tetrahydrofuran (20ml), and the reaction was continued for 1h after the addition was complete. The reaction was quenched by adding 1M hydrochloric acid (10ml), extracted with dichloromethane (40ml×3), the organic phases were combined, dried over anhydrous sodium sulfate, and the solvent was removed to obtain 0.72g of a light yellow solid with a yield of 68.6%. LC-MS(APCI):m / z=118.29(M+1) + .
[0149] Step 2 Synthesis of compound 16.
[0150] 4-Fluoro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com