Dressing prepared by using freeze-dried calcium alginate/vaterite calcium carbonate composite microspheres and preparation method thereof
A technology of composite microspheres and calcium alginate, applied in medical science, bandages, etc., can solve the problems of easy infection of wounds, slow recovery speed, etc., to promote repair and healing, promote repair and healing of damaged tissues, and avoid frequent dressing changes. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A kind of dressing that utilizes freeze-dried calcium alginate / vaterite calcium carbonate composite microspheres to prepare, antibacterial drug in the present embodiment is rifamycin sodium as an example, cell growth factor is mouse basic fibroblast growth factor, The dressing is prepared as follows:
[0041] (1) Co-precipitation method to prepare vaterite calcium carbonate suspension: add Na at a concentration of 50mM to a beaker with a capacity of 100mL 2 CO 3 + 8mg / mL casein 20mL, magnetic stirring at 600rpm, magnet diameter 40mm, at the same time add 20mL of 50mM CaCl in the separatory funnel 2 , Open the cock just above the beaker and add evenly. Magnetic stirring was carried out for 20 min. After the stirring was completed, the carbonate ions and calcium ions in the solution completely reacted to obtain CaCO 3 Suspension, i.e. vaterite calcium carbonate suspension, in which CaCO 3 The concentration is 2.5mg / mL.
Embodiment 2
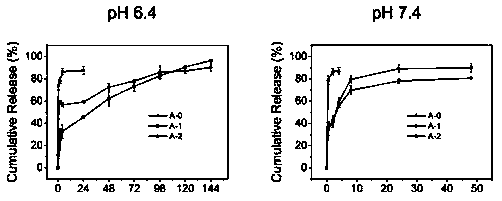
[0053] (1) According to the steps in Example 1, a dressing prepared by using freeze-dried calcium alginate / vaterite calcium carbonate composite microspheres was prepared. In order to facilitate observation and detection, trypan blue (dye ) instead of antibacterial drugs or cell growth factors as loaded drugs, observe the prepared freeze-dried composite microspheres, and its morphology under an optical microscope is as follows figure 2 shown. The light microscope image of the freeze-dried composite microspheres after re-swelling is shown in image 3 shown.
[0054] From figure 2 and image 3 It can be seen from the figure that after lyophilization, the composite microspheres re-swelled to form a hydrogel state, and their spatial structure was more plump and three-dimensional, which did not change much compared with that before lyophilization.
[0055] (2) Referring to the method and steps in (1), trypan blue was used instead of antibacterial drugs or cell growth factors a...
Embodiment 3
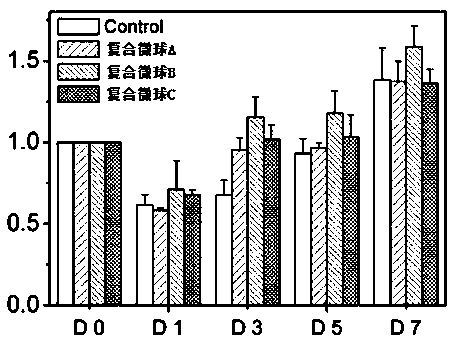
[0059] The antibacterial effect of the composite microspheres loaded with drugs was tested as follows:
[0060] (1) Staphylococcus aureus was inoculated in the culture medium (LB, 1% peptone, 0.5% yeast extract and 1% NaCl), and shaken at 200rpm at 37°C for 24h. Take a portion of the bacterial suspension and dilute it using the gradient method. The bacterial concentration after counting is 8*10 8 CFU / mL. Draw 100 μL and spread evenly on the LB agar medium in the 90mm Petri dish.
[0061] (2) Taking composite microsphere A prepared in Example 1, composite microsphere A and composite microsphere B mixed at a ratio of 1:1 as the test object, the composite microsphere Place A and composite microsphere C on a 90mm filter paper sheet, then transfer the filter paper sheet to the center of the medium in step (1), and culture for 24 hours. In order to analyze the sustained antibacterial effect of the experimental group, the above-mentioned filter paper pieces were transferred to a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com