A processing-destructed β-conglycinin β subunit antigen region and screening method based on phage display technology
A conglycinin, phage display technology, applied in the fields of immunology, molecular biology, bioinformatics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Preparation of allergen epitope-specific antibodies destroyed by ultra-high pressure treatment
[0026] 1. Ultra-high pressure treatment of β-conglycinin:
[0027] Put β-conglycinin with a concentration of 15 mg / mL in a sterile homogeneous bag, seal and vacuumize, place the sealed homogeneous bag in the processing chamber (23°C) of the ultra-high pressure processing device, and start Boost the pressure at a rate of 250MPa / min. When the pressure rises to 455MPa, keep the pressure for 18 minutes and then release the pressure at a rate of 300MPa / min. The inhibition rate of β-conglycinin antigen after treatment was 49.59%.
[0028] 2. Preparation of allergen epitope-specific antibodies (antigen-absorbed serum) destroyed by ultra-high pressure treatment:
[0029] New Zealand white rabbits were fed with natural β-conglycinin to prepare β-conglycinin polyclonal antibody (product 1). Add excess β-conglycinin treated by ultra-high pressure to the prepared polyclona...
Embodiment 2
[0030] Example 2, β-conglycinin β subunit gene overlapping (overlapping) segmental cloning
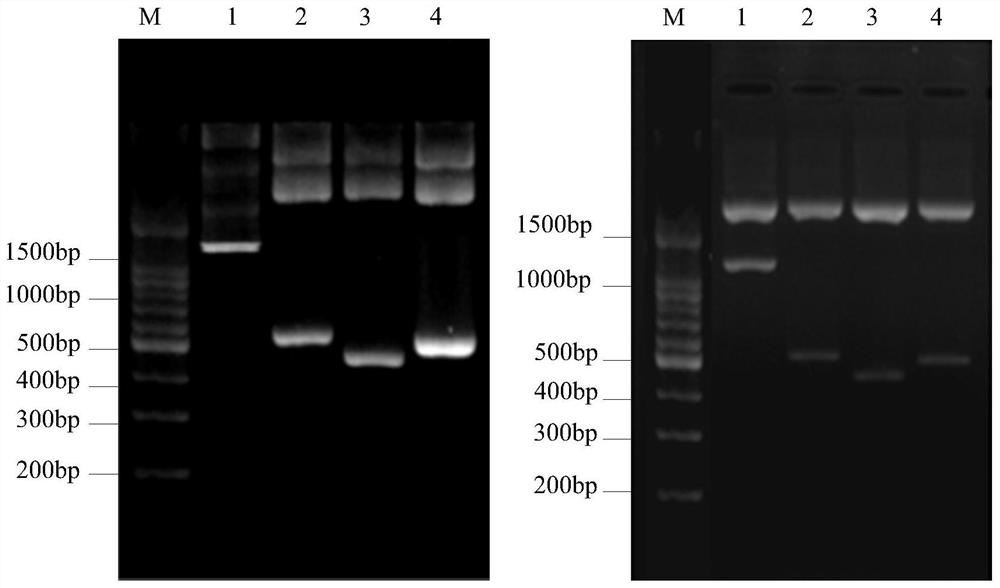
[0031] 1. Cloning of β-conglycinin β subunit gene
[0032] The amino acid sequence of β-conglycinin β subunit (LOC547465) was retrieved from NCBI GenBank database. Login to the PDB database to search for homologous proteins of β-conglycinin β subunit as templates for tertiary structure prediction. Log in to the SWISS-MODEL homology modeling server to predict the tertiary structure of the β-conglycinin β subunit. According to the tertiary structure model of β-conglycinin β subunit, its conformational epitope was predicted in DiscoTope 2.0 web server. According to the predicted position of protein tertiary structure and B cell conformation epitope, the amino acid sequence of β-conglycinin β subunit is divided into 3 segments, and each sequence overlaps with the previous sequence by about 40 amino acid residues (underlined) . The overlapping fragments of the β-conglycinin β subunit se...
Embodiment 3
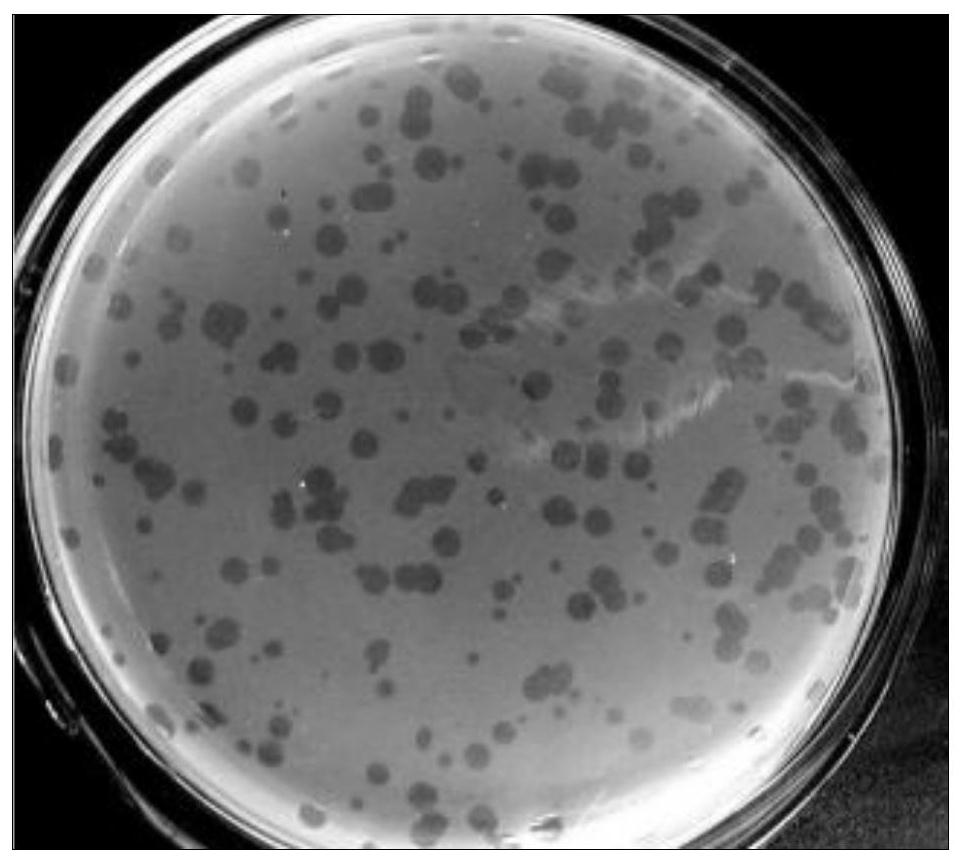
[0064] Example 3, Phage Surface Presentation of Overlapping Fragments
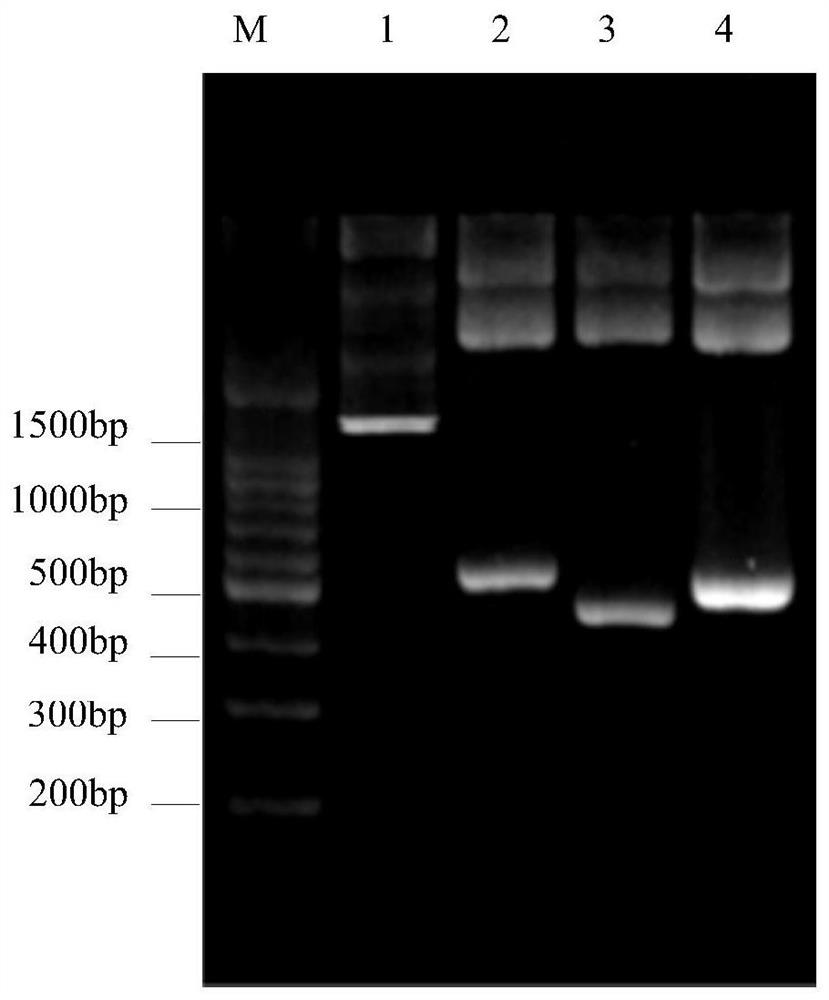
[0065] 1. Enzyme digestion and recovery of β-conglycinin β subunit gene and its fragments
[0066] Use EcoRI and HindIII to perform double enzyme digestion on the recombinant plasmid, and cut out the β-conglycinin β subunit and its fragments. The method is the same as in Example 2. The enzyme digestion products are separated with 2% agarose gel, and the target DNA gel is cut out. Afterwards, the target fragment was recovered with a gel recovery kit.
[0067] 2. Phage packaging
[0068] Ligate the recovered and purified target fragment to the carrier arm at a molar ratio of 1:1-3:1 (insert:vector), and the connection system is as follows:
[0069]
[0070] Mix by pipetting up and down, then incubate at 16°C for 3-16h or store at 4°C until use. Add 5 times the volume of packaging extract to the ligation product, mix gently with the pipette tip, incubate at room temperature (22°C) for 2 h, add 9 times t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com