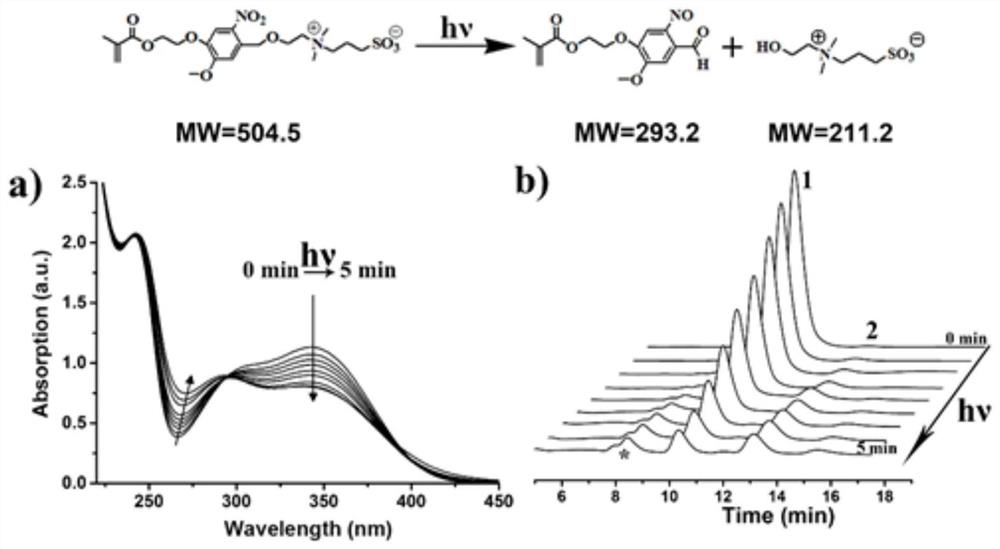
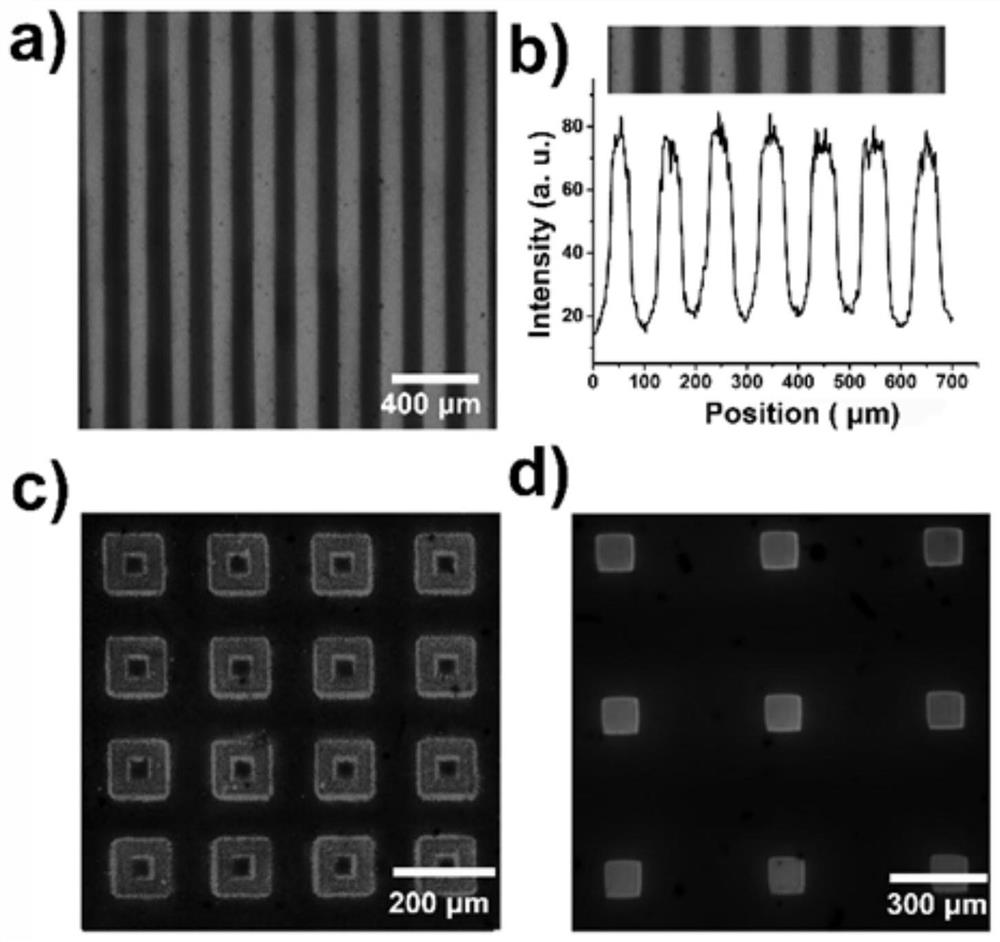
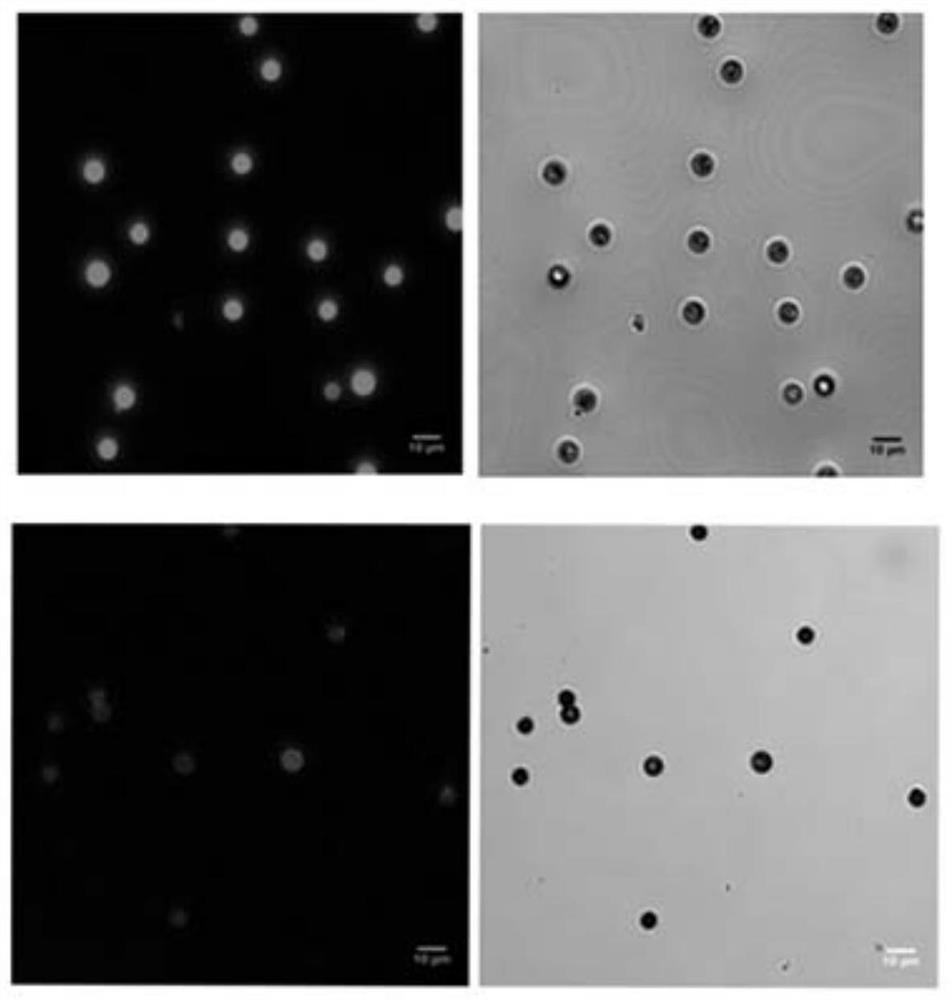
Table of Materials/Methods for the Covalent Immobilization of Bioactive Factors by Photogenerated Aldehyde/Kone Groups at the Interface
A bioactive factor, covalent immobilization technology, applied in the biological field, can solve the problems of low efficiency, easy quenching of free radicals, long reaction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: Synthesis of photosensitive functional molecule 6
[0083]
[0084] Synthetic route of photosensitive functional molecule 6
[0085] (1) Synthesis of compound 1
[0086] Add vanillin (15g, 0.1mol) in a 500mL single-necked round bottom flask, dissolve it with 300mL of acetonitrile, slowly add potassium carbonate (16.35g, 1.2mol) and potassium iodide (19.62g, 1.2mol) into the flask while stirring , to prevent stirring, heated to reflux at 90°C, and reacted overnight under the protection of argon. TLC (PE:EA=1:1) detects the progress of the reaction. After the reaction is over, cool the system to room temperature, filter with suction, spin dry the filtrate, dissolve the solid in ethyl acetate, wash with water to remove soluble salts, spin dry the solid with 95% ethanol After recrystallization, after cooling and crystallization was complete, suction filtration was obtained to obtain a white solid, which was dried in an oven to obtain 20.3 g of solid, name...
Embodiment 2
[0121] Embodiment 2: Synthesis of photosensitive functional molecule 8
[0122]
[0123] Synthetic route of photosensitive functional molecule 8
[0124] (1) Synthesis of Compound 7
[0125] Add compound 6 (2g, 0.006mol), N,N-dimethyl-3-propion hydrochloride (1g, 0.01mol), carbodiimide (1.9g, 0.01mol) in 100mL round bottom flask, 4-Lutidine (1.2 g, 0.01 mol), dissolved in 50 mL of dehydrated DCM, stirred at room temperature, and reacted overnight under the protection of argon. TLC (DCM 1 mL plus three drops of MeOH) was used to detect the progress of the reaction. After the reaction, wash with water to remove water-soluble carbodiimide, and purify with silica gel chromatography column (DCM:MeOH=0.5%~1%) to obtain 1.8g of viscous liquid, namely compound 7, with a yield of 70.6%, avoiding wavelength 365nm light direct preservation.
[0126] The physicochemical parameters of compound 7 are:
[0127] 1 H NMR (400MHz, CDCl 3 ),δ(ppm):7.78(s,1H),7.04(s,1H),6.14(s,1H),5.60(...
Embodiment 3
[0136] Example 3: Synthesis of photoresponsive functional molecule 12
[0137]
[0138] Synthetic route of photosensitive functional molecule 12
[0139] (1) Synthesis of compound 9
[0140] Add tetraethylene glycol (20 g, 0.1 mol) into a 100 mL round bottom flask, add 50 ml THF to dissolve, add 2 mL TEA, and stir under ice-bath conditions. Under the protection of argon, a THF solution of p-toluenesulfonyl chloride (1.9 g, 0.01 mol) was added dropwise. After the dropwise addition, the ice bath was removed to continue stirring, and the reaction was detected by TLC. After the reaction, the solvent was spin-dried, dissolved in dichloromethane, washed with water, and the organic phase was separated and dried over anhydrous sodium sulfate. After spin-drying, the product was purified by silica gel column chromatography, namely compound 9.
[0141] The physicochemical parameters of compound 9 are:
[0142] 1 H NMR (400MHz, CDCl 3 ),δ(ppm):7.75(2H,d,J=8.2Hz,),7.31(2H,d,J=8Hz,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com