A kind of method of green synthetic benproperine phosphate intermediate
A technology of benproperine phosphate and green synthesis, applied in the direction of organic chemistry, etc., to achieve the effects of simplified production process, easy operation and improved safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
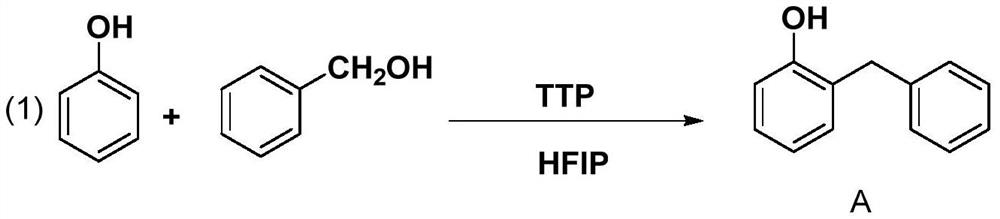
[0017] Embodiment 1: the synthesis of o-benzylphenol
[0018]
[0019] In a 500ml three-necked flask, sequentially add 1,1,3,3-tetrafluoromethanesulfonylpropene (TTP) (90.63g, 159mmol), phenol (10g, 106mmol), benzyl alcohol (13.78g, 127.2mmol) , add 200ml hexafluoroisopropanol (HFIP) as reaction solvent. Heating to 100°C for 24 hours of reaction, after the reaction, the solvent, phenol, and benzyl alcohol were distilled under reduced pressure. Drying; to obtain the target product A, 18.9 g of a white solid was obtained, and the yield was 97.0%. MS(EI):m / z:184.0888([M] + ).
Embodiment 2
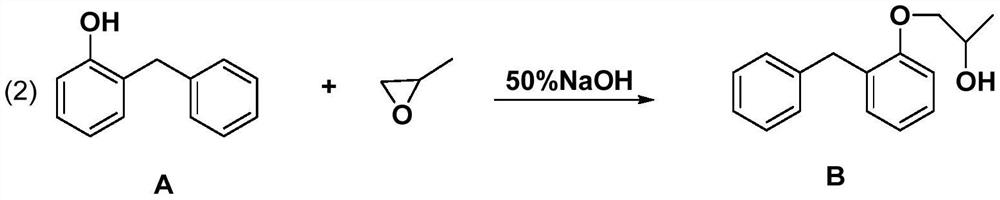
[0020] Embodiment 2: the synthesis of (2-benzylphenoxy)-2-propanol
[0021]
[0022] In a 500ml three-necked flask, sequentially add 36g of compound A (18.9g, 103mmol) 50% NaOH aqueous solution (500g sodium hydroxide + 500g purified water), stir and heat up to 65°C, slowly add propylene oxide (7.160g, 123.6mmol) , reacted for 1 h, then slowly raised the temperature to 100 °C, reacted for 3 h, and the reaction was completed, adding acid to adjust the pH of the solution to 7, repeated extraction with toluene, combined the extraction phases, spin-dried the solvent under reduced pressure, and recrystallized with ethanol to obtain a light yellow solid B ( 23.5g, yield 100%). ;
Embodiment 3
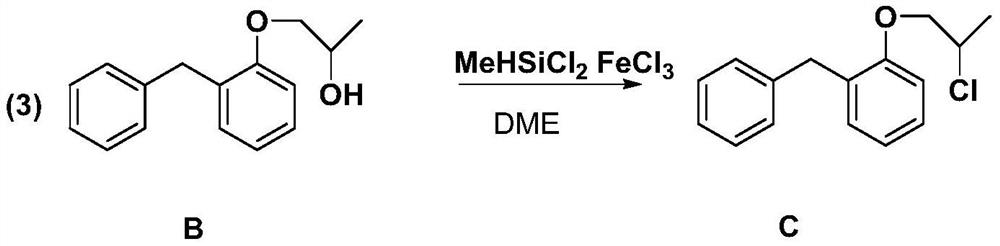
[0023] Example 3: (2-benzylphenyl) 2-chloropropyl ether
[0024]
[0025] In a 500ml three-necked flask, add compound B (23.5g, 103mmol), methyl dichlorosilane (14.22g, 123.6mmol), anhydrous ferric chloride (1.67g, 10.3mmol), ethylene glycol dimethyl ether 200ml as The reaction solvent was heated to 84° C. for reflux reaction for 4 hours. After the reaction, the solvent was removed under reduced pressure and dried. Recrystallization from ethyl acetate afforded white solid C (23.8 g) with a yield of 88.59%. MS(EI):m / z:260.0968([M] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com