3-(2-benzoxazole)coumarin formamide compound, and preparation method and application thereof
A technology of coumarin amide and benzoxazole, which is applied in organic chemistry, optics, instruments, etc., can solve the problem of less research on coumarin base, and achieve the effect of great implementation value, simple process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
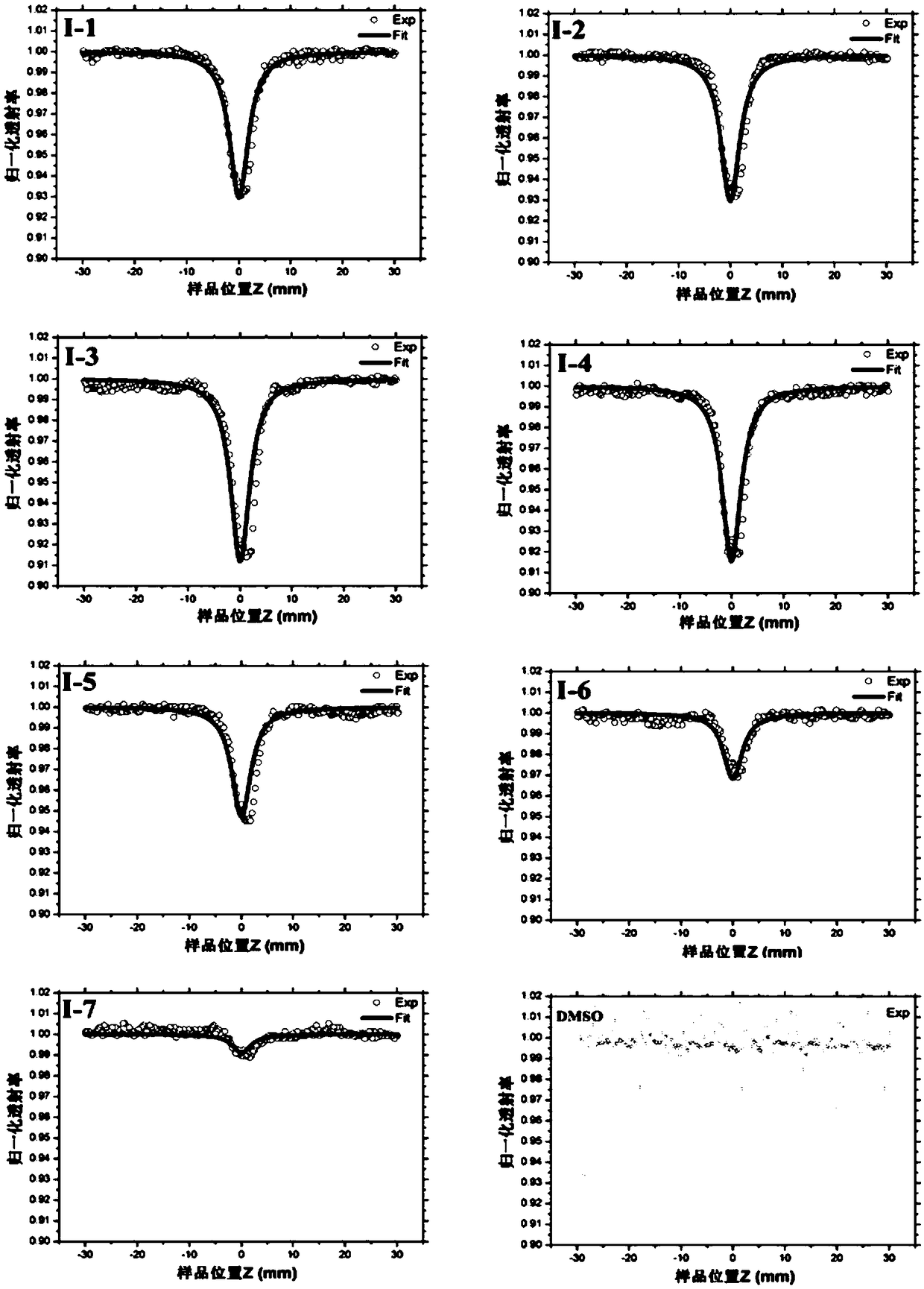
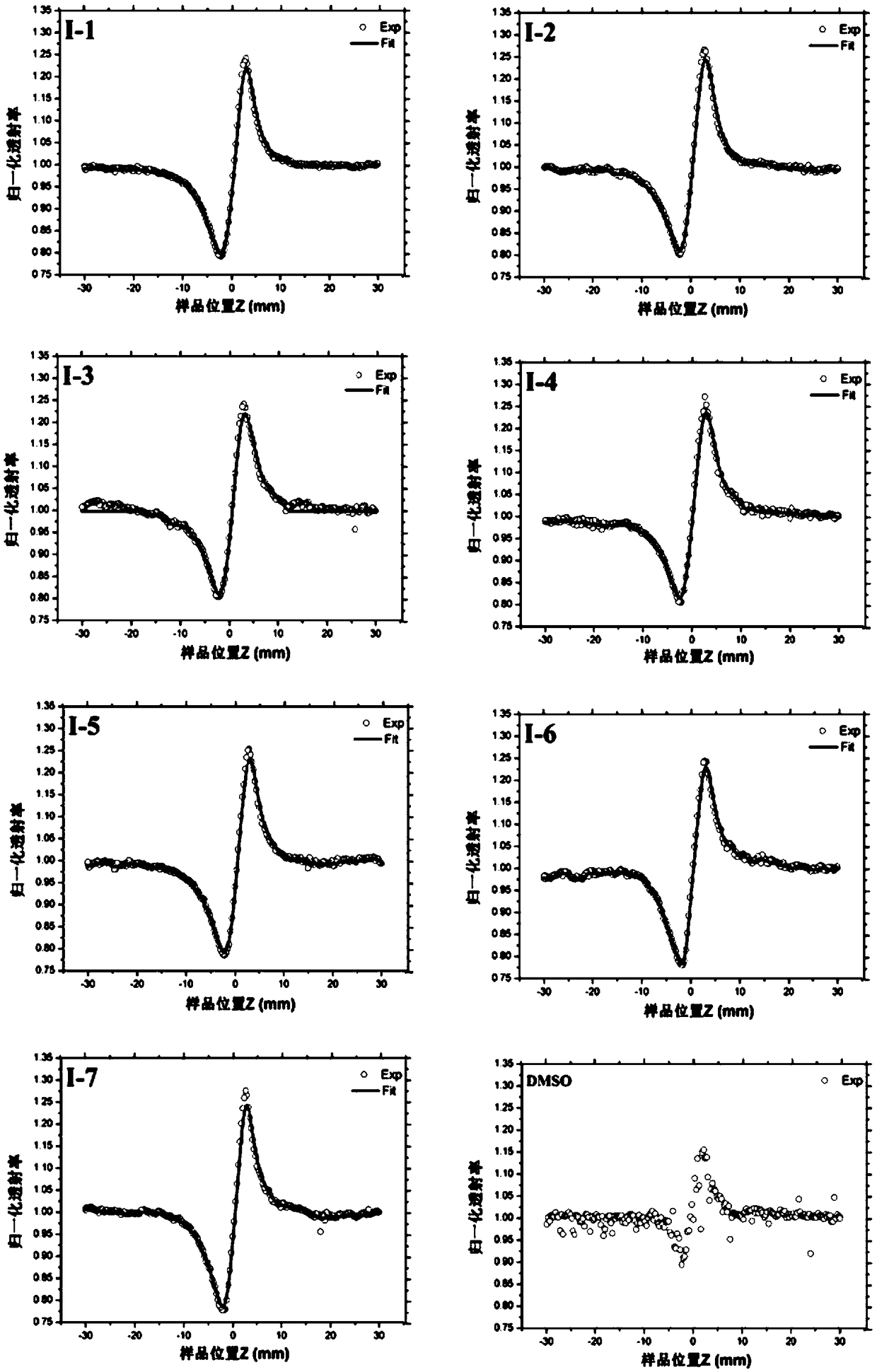
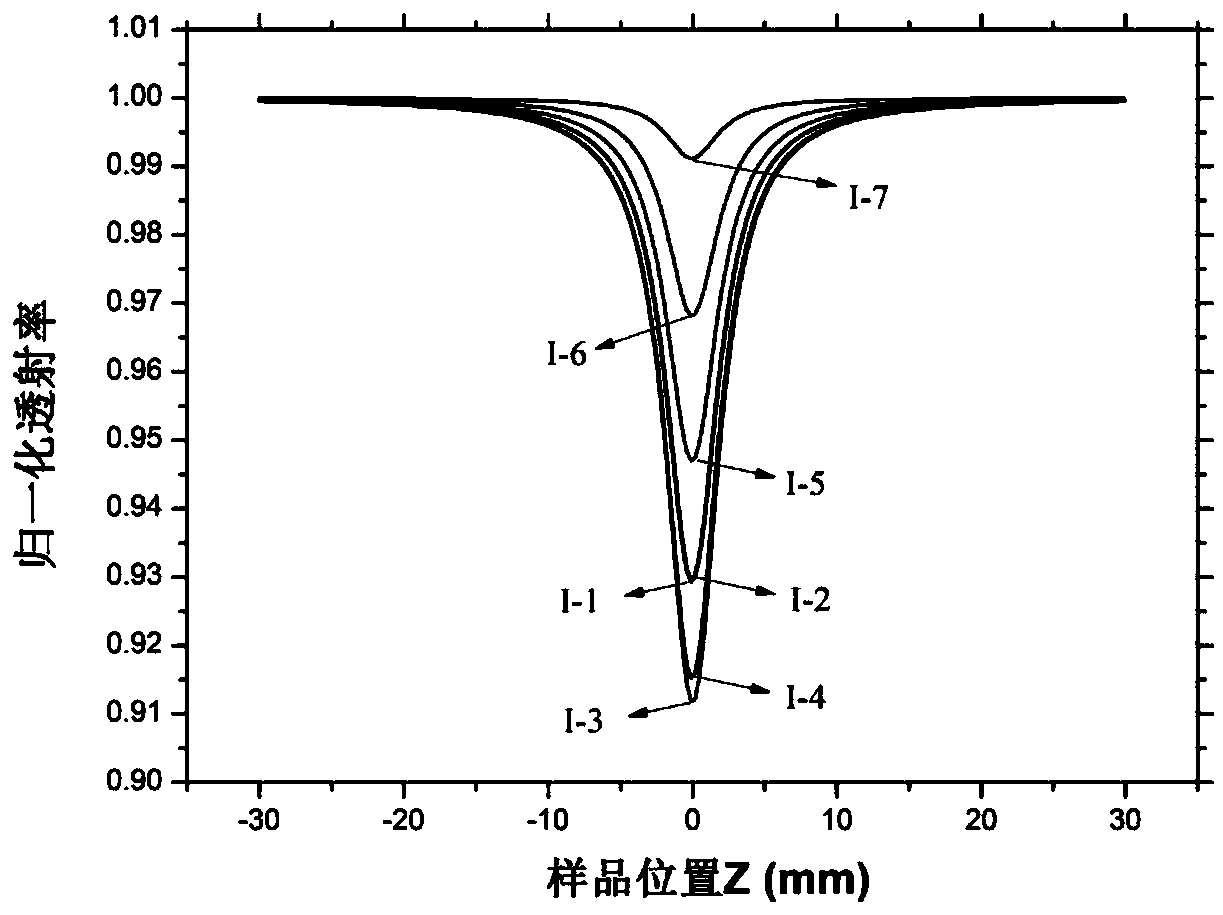
Image
Examples
Embodiment 1
[0061] Preparation of 2-(2-oxo-2H-coumarin-3-yl)benzo[d]oxazole-5-carboxylic acid (abbreviated as compound IIIa): according to the amount of substance, formula (II): cyanoacetic acid Ethyl: 4-carboxy-2-aminophenol: organic acid is 1.0: 1.0: 1.1: 0.3 feed, where formula (II) is salicylaldehyde (ie R 1 Is hydrogen), the feed mass is 61.1g, ethyl cyanoacetate is 56.6g, 4-carboxy-2-aminophenol is 84.2g, the organic acid is p-toluenesulfonic acid, and the feed mass is 25.8g; alcohol solvent A is ethanol 240mL, The volume dosage is about 4 times the mass of formula (II) (mL / g).
[0062] Add salicylaldehyde, ethyl cyanoacetate, 4-carboxy-2-aminophenol, ethanol and p-toluenesulfonic acid into the reaction flask, and heat to reflux for reaction. TLC (Thin Layer Chromatography) tracking, after the reaction was completed, cooling in an ice bath, a large amount of light yellow solid precipitated, filtered, the filter cake was rinsed with water, and dried to obtain 102.3 g of the product, nam...
Embodiment 2
[0064] According to the quantity ratio of the substance, the formula (II): ethyl cyanoacetate: 4-carboxy-2-aminophenol: organic acid is 1.0: 1.2: 1.2: 0.1 feeding, where the formula (II) is salicylaldehyde, and the feeding quality is 61.1 g, ethyl cyanoacetate 67.9g, 4-carboxy-2-aminophenol 91.9g, organic acid is benzoic acid, feed mass is 6.1g; alcohol solvent A is n-propanol 360mL, and its volume dosage is formula (II ) About 6 times the mass (mL / g).
[0065] Other operations were the same as in Example 1, to obtain 99.6 g of the product, namely compound IIIa, with a yield of 64.8%. Combined with the characterization data, compound IIIa is a compound of formula (III), where R 1 Is hydrogen.
Embodiment 3
[0067] Preparation of 2-(7-(diethylamino)-2-oxo-2H-coumarin-3-yl)benzo[d]oxazole-5-carboxylic acid (abbreviated as compound IIIb): according to the amount of substance Formula (II): ethyl cyanoacetate: 4-carboxy-2-aminophenol: organic acid is 1.0: 0.9: 0.9: 0.6 feeding, wherein formula (II) is 4-diethylamino salicylaldehyde (ie R 1 Is diethane substituted amino), the feed mass is 96.6g, ethyl cyanoacetate is 50.9g, 4-carboxy-2-aminophenol is 68.9g, the organic acid is acetic acid, and the feed mass is 18.0g; the alcohol solvent A is iso For 300 mL of propanol, its volumetric dosage is about 3 times the mass of formula (II) (mL / g).
[0068] Other operations were the same as in Example 1, to obtain 116.5 g of the product, namely compound IIIb, with a yield of 68.4%. Combined with the characterization data, compound IIIb is a compound of formula (III), where R 1 Amino substituted with diethane.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com