Indole alkaloid with anti-tumor activity as well as preparation method and application thereof
An indole alkaloid, anti-tumor activity technology, applied in the field of microbial engineering technology and pharmacology, can solve the problems of drug resistance, restricting the treatment effect and quality of life of leukemia patients, low treatment efficiency, etc. The effect of short cycle and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Liquid fermentation and feeding of mantis intestinal bacteria IFB-T01 (Daldinia eschscholzii)
[0030] The bacterial block of Daldinia eschscholzii IFB-TL01 derived from the intestinal bacteria of the activated mantis mantis was inoculated in 1L Erlenmeyer flasks, each containing 400mL of malt culture medium, inoculated 10 flasks on a shaker, and cultured at 200rpm, 28‐30℃ for 2 ‐3 days, as the seed liquid, and then inoculate the seed liquid into a new malt medium (400 mL / bottle x 200 bottles) with an inoculum amount of 20 mL each, and continue to cultivate for 2 days at 200 rpm and 28‐30 °C. Indole-3-carbinol was fed at 24, 48, and 72 hours respectively until the final concentration in the solution was 1.0 mM, and the fermentation was continued for 10 days at 200 rpm and 28-30°C.
Embodiment 2
[0031] Embodiment 2: Extraction and separation of indole alkaloids
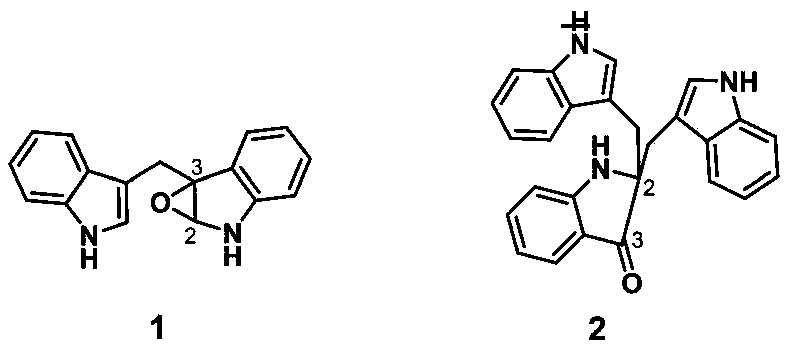
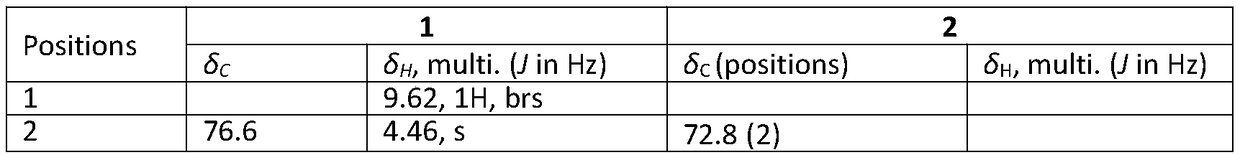
[0032] In Example 1, the fermented liquid was filtered through gauze, the filtrate was extracted with ethyl acetate, and concentrated by centrifugation to obtain black extract A (146g); the extract A was subjected to silica gel column chromatography segmentation, and petroleum ether: acetone (volume ratio: 100: 0, 100:5, 10:1, 5:1, 3:1, 2:1, 1:1) for gradient elution to obtain 7 eluted fractions A1-A7;
[0033] Take segment A3 (petroleum ether: acetone = 10:1 elution site) and continue to use petroleum ether: acetone (volume ratio 100:2 → 1:1) gradient elution to obtain F1-F77 fractions. Take F3 (petroleum ether: acetone volume ratio 20:1 elution fraction) through Sephadex LH-20 column chromatography (methanol elution), high pressure preparative liquid phase [chromatographic column: ODS-2Hy0persil columns (5μm, 250×10mm)] MeOH / H 2 O (70:30) was eluted and purified to obtain compound 1 (2 mg).
[0034] And ...
Embodiment 3
[0043] Embodiment 3: MTT method (3-(4,5-dimethylthiazole-2)-2,5-diphenyltetrazolium bromide salt method) measures the antitumor activity of compounds 1 and 2
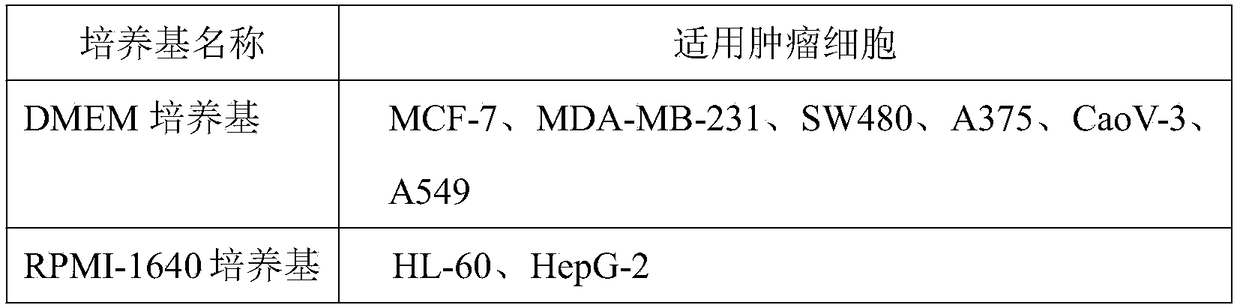
[0044] 1. Medium configuration: the medium contains 10% newborn calf serum, penicillin 100U / ml and streptomycin 100U / ml.
[0045]
[0046] 2. Cell culture: Inoculate human breast cancer cells SW480, HL-60, HepG-2, A375, MCF-7, CaoV-3, A549 and MDA-MB-231 cells into culture flasks containing corresponding medium, Place at 37°C, 5% CO 2 Culture in an incubator under the condition of relative saturated humidity, pass passage every 3-5 days.
[0047] 3. Test drug treatment: Dissolve compounds 1 and 2 in an appropriate amount of DMSO (final concentration not exceeding 0.5%), then dilute it into a 10-fold working solution with complete medium, and store it at 4°C for later use.
[0048] 4. MTT colorimetric method to detect the effect of drugs on tumor cell proliferation: select tumor cells SW480, HL-60, HepG-2, A375, MCF...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com