Preparation method for metal single atom/phosphor doping carbon nitride photocatalyst
A carbon nitride light and phosphorus doping technology, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, non-metallic elements, etc., can solve the problem of narrow forbidden energy band, achieve low preparation cost, and improve visible light absorption Utilization, the effect of excellent photolysis water hydrogen production activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
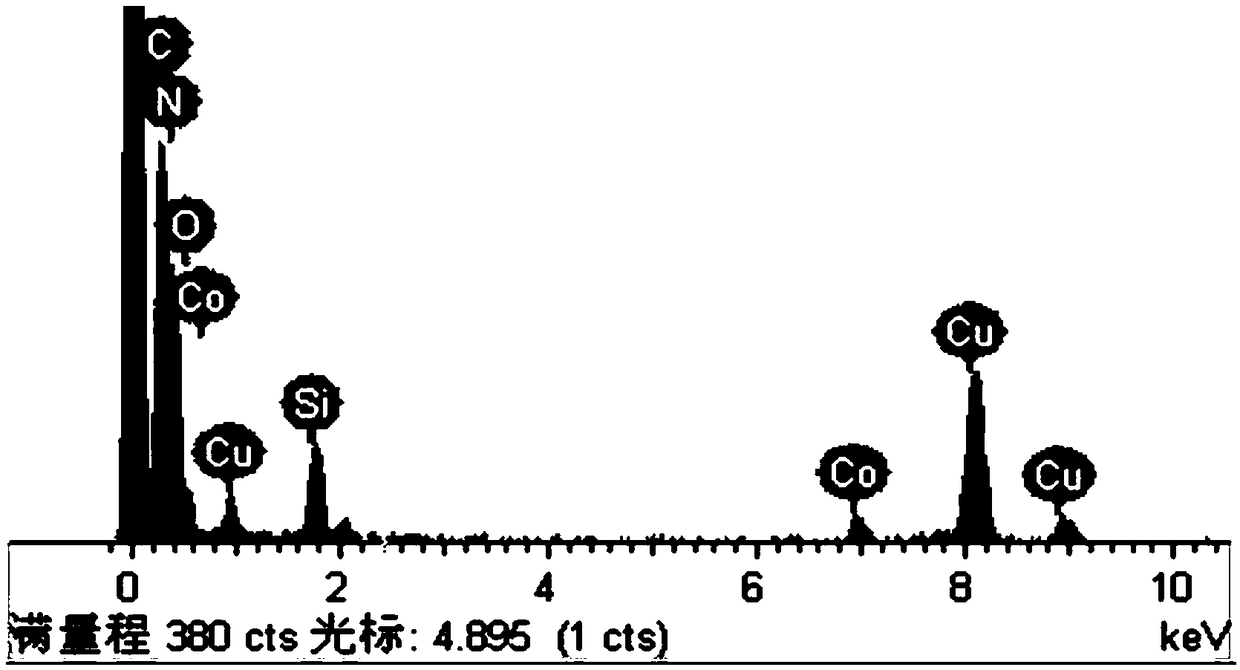
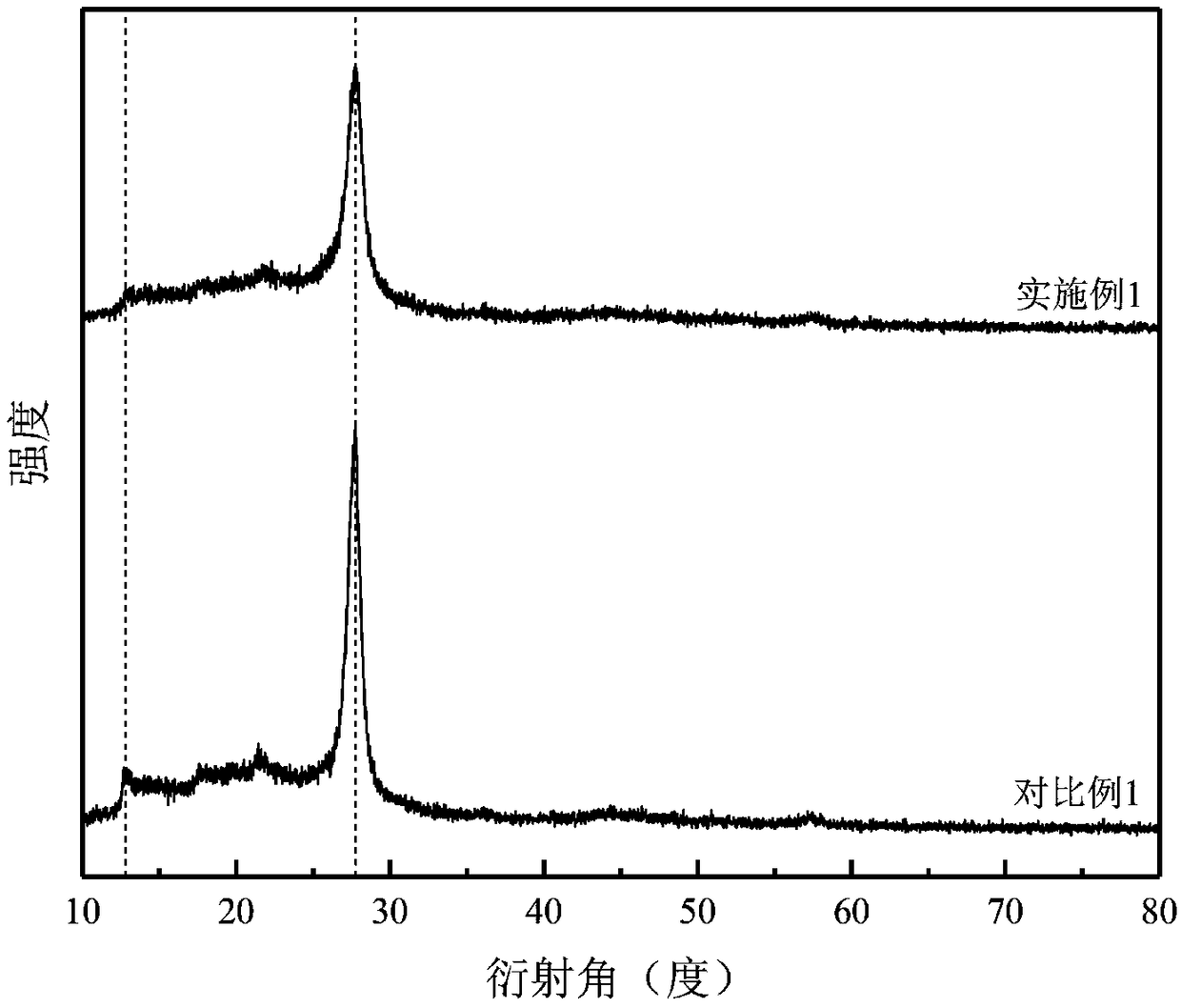
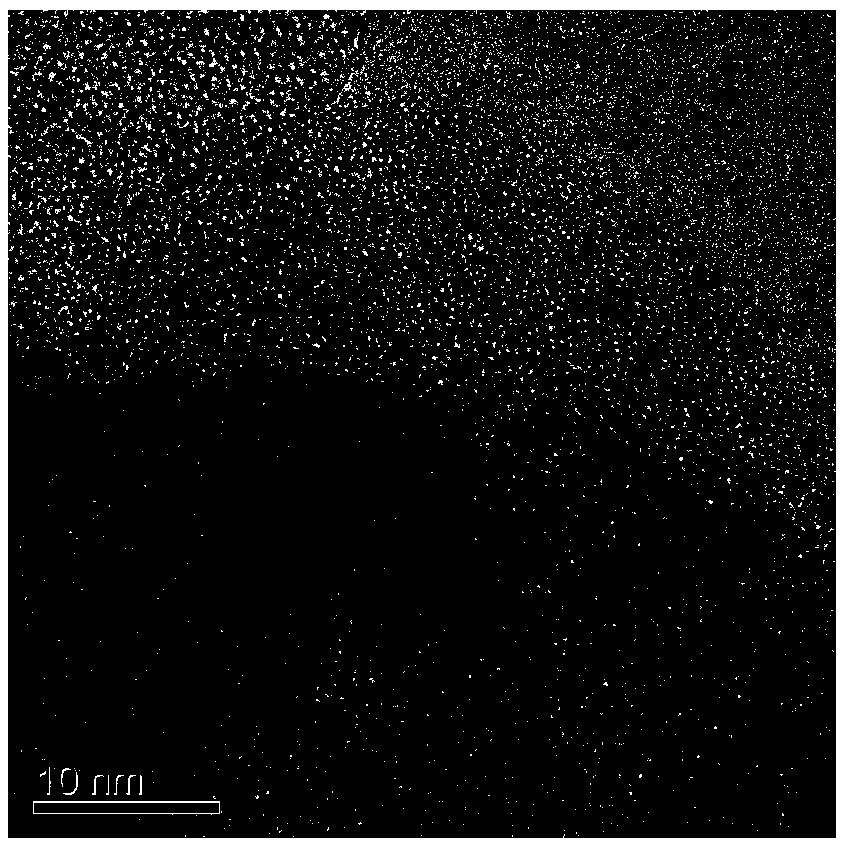
[0027] Mix 10 g of urea and 0.2 g of cobalt chloride in ethanol uniformly, reflux at 45° C. for 3 h, and then evaporate the solvent to dryness to obtain urea-cobalt complex. Mix 5g of urea-cobalt complex and 4g of melamine evenly, place in a tube furnace, raise the temperature to 580°C under an argon atmosphere, keep the temperature at a constant temperature for 3 hours, and after natural cooling, monoatomic cobalt / carbon nitride is obtained. After the monoatomic cobalt / carbon nitride and sodium hypophosphite are mixed uniformly at a mass ratio of 1:2, they are placed in a tube furnace and heated to 300°C under an argon atmosphere, and the temperature is kept constant for 2 hours. After natural cooling, the obtained solid was washed several times and dried to obtain monoatomic cobalt / phosphorus doped carbon nitride.
Embodiment 2
[0029] Mix 20g of urea with 40mg of silver nitrate, 40mg of ferric nitrate and 40mg of copper acetate in water, reflux at 60°C for 2 hours, and then evaporate the solvent to dryness to obtain a urea silver+iron+copper complex. Mix 10g of urea silver+iron+copper complex with 4g of dicyandiamide evenly, put it in a tube furnace, raise the temperature to 500°C under an argon atmosphere, keep the temperature at a constant temperature for 10h, and after natural cooling, monoatomic silver+iron is obtained + Copper / Carbon Nitride. Monoatomic silver+iron+copper / carbon nitride and magnesium hypophosphite are mixed evenly at a mass ratio of 1:0.5, and placed in a tube furnace under an argon atmosphere to raise the temperature to 400°C and keep the temperature constant for 1h. After natural cooling, the obtained solid was washed several times and dried to obtain monoatomic silver+iron+copper / phosphorus doped carbon nitride.
Embodiment 3
[0031] Mix 15g of urea with 0.4g of ferric chloride and 0.4g of nickel nitrate in methanol, reflux at 25°C for 24h, and then evaporate the solvent to dryness to obtain urea iron+nickel complex. Put 10g of urea-iron+nickel complex in a tube furnace, raise the temperature to 650°C under a mixed gas atmosphere of nitrogen and argon, and keep the temperature for 1h to obtain monatomic iron+nickel / carbon nitride. The mixture of monoatomic iron+nickel / carbon nitride and potassium hypophosphite, magnesium hypophosphite and ammonium hypophosphite is mixed uniformly at a mass ratio of 1:5, and placed in a tube furnace in an argon atmosphere Lower the temperature to 250°C and keep the temperature constant for 4h. After natural cooling, the obtained solid was washed several times and dried to obtain monoatomic iron+nickel / phosphorous doped carbon nitride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com