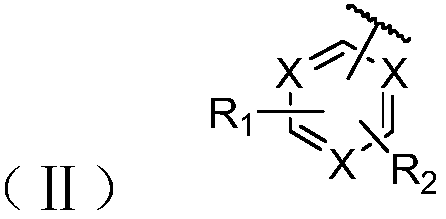
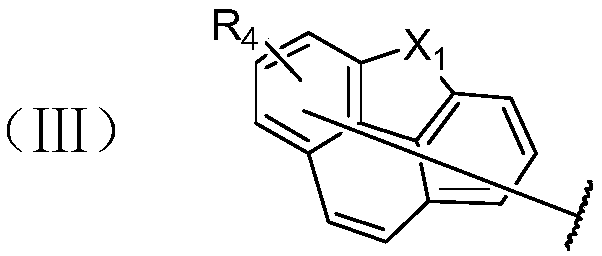
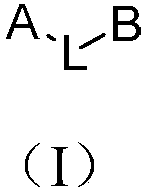Phenanthrene-containing compound and organic light-emitting device thereof
A technology of electroluminescent devices and compounds, applied in the field of phenanthrene-containing compounds and organic electroluminescent devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Preparation of compound 1
[0074]
[0075] Preparation of compound 1-3
[0076] Under argon atmosphere, compound 1-1 (5.39g, 15mmol), p-bromoiodobenzene (4.05g, 14.3mmol), tetrakis(triphenylphosphine)palladium (0.35g, 0.3mmol), toluene (43ml), carbonic acid Sodium aqueous solution (2M, 21ml) was added to the flask, refluxed for 8 hours, cooled to room temperature, extracted with toluene, the organic phase was washed with saturated brine, and the organic phase was dried and purified by column chromatography to obtain bromide 1-3 4.76g, yield 82%.
[0077] Preparation of compound 1
[0078] The obtained bromide 1-3 (7.74g, 20mmol), pinacol biborate (6.22g, 24mmol), 1,1'-bis(diphenylphosphine)-ferrocene-palladium dichloride (II ) Dichloromethane complex (0.49g, 0.6mmol), potassium acetate (5.90g, 60mmol) and 79ml of toluene were reacted under reflux for 16 hours. After cooling, 26ml of water was added and stirred for 30 minutes. The organic phase was separated and passed throug...
Embodiment 2
[0080] Preparation of compound 20
[0081]
[0082] Preparation of compound 20
[0083] Replace 1-4 in Example 1 with 20-4 as shown above to obtain compound 20. Mass spectrum m / z: 757.35 (calculated value: 757.36). Theoretical element content (%) C 56 H 43 N 3 : C, 88.74; H, 5.72; N, 5.54 Measured element content (%): C, 88.75; H, 5.73; N, 5.55 The above confirmed product is the target product 20.
Embodiment 3
[0085] Preparation of compound 36
[0086]
[0087] Preparation of compound 36
[0088] Replace 1-4 in Example 1 with 36-4 as shown above to obtain compound 36. Mass spectrum m / z: 703.30 (calculated value: 703.31). Theoretical element content (%) C 52 H 37 N 3 : C, 88.73; H, 5.30; N, 5.97 Measured element content (%): C, 88.72; H, 5.31; N, 5.98. The above confirmed that the obtained product is the target product 36.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com