Alkali tungsten bronze nanorod and preparation method and application thereof
A technology of alkali tungsten bronze and nanorods, which is applied in the field of nanomaterials, can solve the problems of large particle size, poor reproducibility, and many impurities of alkali tungsten bronze products, and achieve the effects of unindustrialization, large output, and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 0.02mol tungsten powder into a 500ml beaker, add 30mL H 2 o 2 (30%), and stirred in cold water to fully react the tungsten powder. After about 1 hour, the tungsten powder was completely reacted, filtered, stirred and heated in a water bath at 80°C, the solution changed from transparent yellowish to yellow sol, and after about 5 hours, about 10mL yellow sol. Add 0.18384g polyethylene glycol 600 (5% tungsten powder mass) to the sol, stir evenly, add 0.066 mol CsCl, stir evenly, and obtain a yellow gel. The gel was dried in a drying oven at 80°C to obtain the precursor. The precursor was placed in a tube furnace, and the temperature was raised to 600° C. for calcination for 2 h under an argon atmosphere to obtain cesium tungsten bronze nanorods.
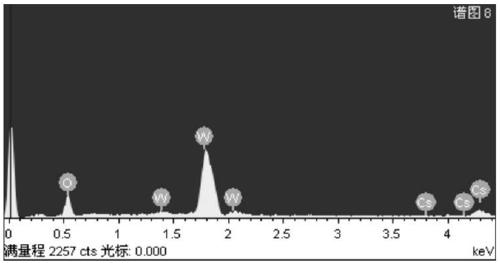
[0032] The obtained powders were characterized by X-ray diffractometer, field emission scanning electron microscope and energy dispersive spectrometer.
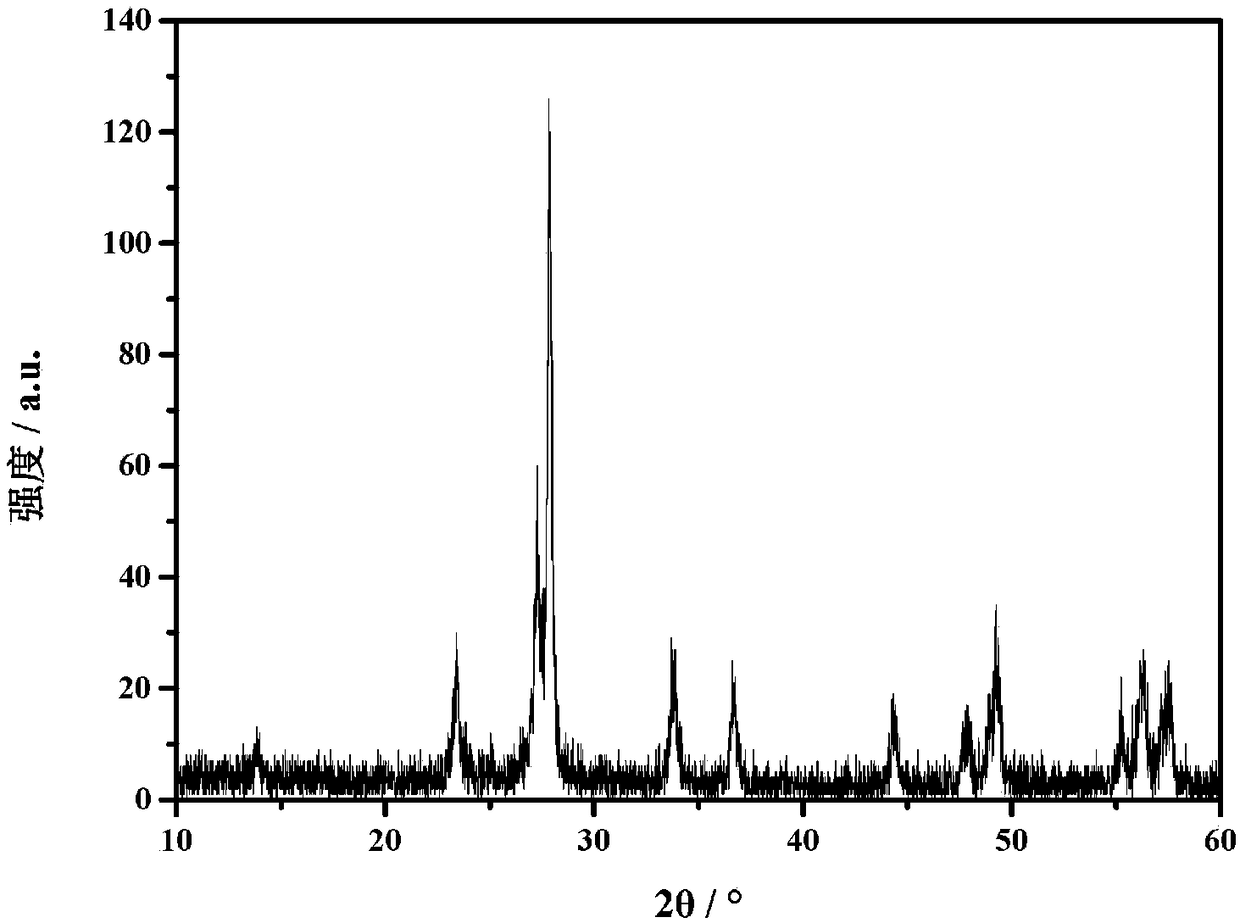
[0033] figure 1 The X-ray diffraction spectrum of the cesium tun...
Embodiment 2
[0035] Weigh 0.02mol tungsten powder into a 500ml beaker, add 30mL H 2 o 2 (30%), and stirred in cold water to fully react the tungsten powder. After about 1 hour, the tungsten powder was completely reacted, filtered, stirred and heated in a water bath at 80°C, the solution changed from transparent yellowish to yellow sol, and after about 5 hours, about 10mL yellow sol. Add 0.18384g polyethylene glycol 600 (5% tungsten powder mass) to the sol, stir well, add 0.066 mol RbCl, stir well, and get a yellow gel. The gel was dried in a drying oven at 80°C to obtain the precursor. Put the precursor in a tube furnace, and raise the temperature to 600° C. for calcination for 2 hours under an argon atmosphere to obtain rubidium-tungsten bronze nanorods.
[0036] The obtained powders were characterized by X-ray diffractometer, field emission scanning electron microscope and energy dispersive spectrometer.
[0037] Figure 4 The X-ray diffraction spectrum of the rubidium tungsten bron...
Embodiment 3
[0039] Weigh 0.02mol tungsten powder into a 500ml beaker, add 30mL H 2 o 2 (30%), and stirred in cold water to fully react the tungsten powder. After about 1 hour, the tungsten powder was completely reacted, filtered, stirred and heated in a water bath at 80°C, the solution changed from transparent yellowish to yellow sol, and after about 5 hours, about 10mL yellow sol. Add 0.18384g polyethylene glycol 600 (5% tungsten powder mass) to the sol, stir evenly, add 0.033mol CsCl and 0.033mol RbCl, stir evenly, and obtain a yellow gel. The gel was dried in a drying oven at 80°C to obtain the precursor. Place the precursor in a tube furnace, and in an argon atmosphere, raise the temperature to 600° C. for calcination for 2 hours to obtain cesium-rubidium co-doped tungsten bronze nanorods.
[0040] The obtained powders were characterized by X-ray diffractometer, field emission scanning electron microscope and energy dispersive spectrometer.
[0041] Figure 7 The X-ray diffractio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com