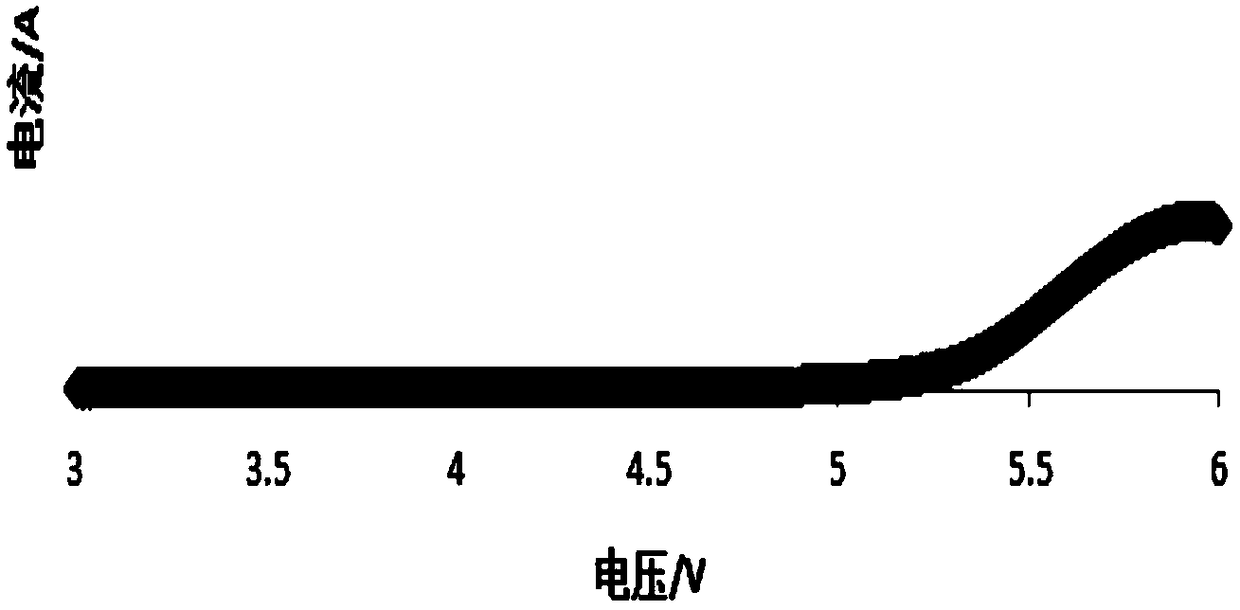
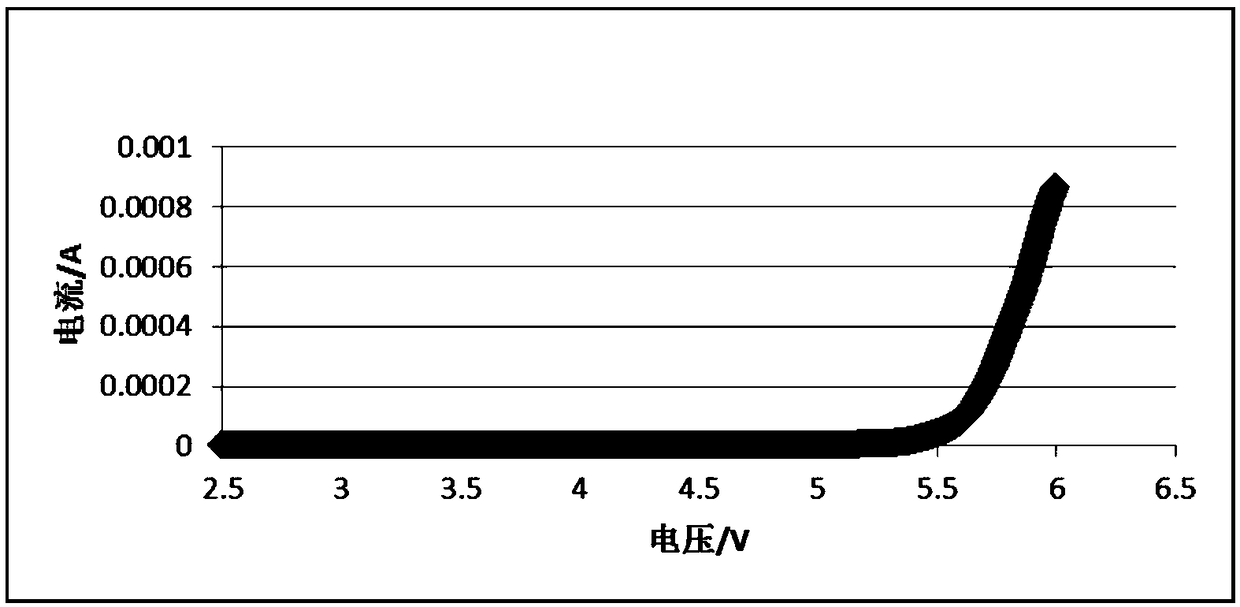
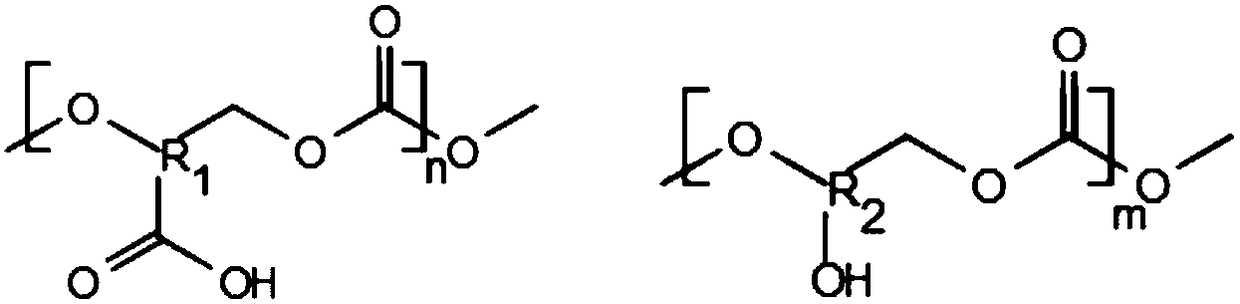A preparation method of an all-solid-state polymer electrolyte and an all-solid-state polymer battery
An all-solid-state polymer and electrolyte technology, applied in non-aqueous electrolyte batteries, electrolyte battery manufacturing, solid electrolytes, etc., can solve problems such as reducing the mechanical strength and energy density of electrolytes, reducing the mobility of molecular chains, and easily changing the flexibility of molecular chains. , to achieve good application potential, improve ionic conductivity at room temperature, and improve mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the all-solid electrolyte of the present invention is as follows:
[0035] S1. Add 5-70 parts by mass of polycarbonate monomer and 0.5-6 parts by mass of carboxyl or hydroxyl polycarbonate monomer into the reactor for polymerization reaction, and obtain polymer A after purification;
[0036]The polycarbonate monomer can be one or more of diphenolic propane and trimethylene carbonate, and the carboxyl or hydroxyl polycarbonate monomer can be 2,2-bis(4-hydroxyphenyl)propionic acid, 5-methyl-2-oxo-1,3-dioxane-5-carboxylic acid, 5-hydroxy-1,3-dioxan-2-one, 5-ethyl-5-(hydroxymethyl )-1,3-dioxan-2-one or several; polycarbonate monomers and carboxyl- or hydroxyl-containing polycarbonate monomers can be produced by phosgene method, transesterification method or thermocatalytic method Carry out the polymerization reaction, the polymer A that obtains is a kind of carboxyl or carboxyl polycarbonate polymer, wherein a kind of polymer has polycarbonate str...
Embodiment 1
[0046] S1. Add 5 parts by mass of trimethylene carbonate and 0.5 parts by mass of 5-methyl-2-oxo-1,3-dioxane-5-carboxylic acid into the reactor, and perform polymerization by thermal catalytic polymerization , continue to feed nitrogen into the reactor and continue to stir at a rotating speed of 200r / min, then add 30 parts by mass of toluene (solvent), 0.01 parts by mass of stannous isooctanoate (catalyst), and react at 100°C for 60min to obtain Polymer A ; The catalyst used in this step can also be tributyltin oxide or tin acetate or rare earth or biological enzyme except that it can be stannous isooctanoate;
[0047] S2. Add 80 parts by mass of polyethylene glycol methyl ether methacrylate (molecular weight 300) and 0.5 parts by mass of polyethylene glycol methyl methacrylate (molecular weight 2500) to 300 parts by mass of toluene, continue to pass nitrogen, and use 500rr Stir at a speed of 1 / min, then add 30 parts by mass of lithium bistrifluoromethanesulfonyl imide (LiTFSI...
Embodiment 2
[0056] S1. Add 70 parts by mass of trimethylene carbonate and 6 parts by mass of 5-hydroxy-1,3-dioxan-2-one into the reactor for thermocatalytic polymerization reaction, and continue to pass nitrogen into the reactor and continue to Stir at a speed of 800r / min, then add 60 parts by mass of toluene and 0.9 parts by mass of tributyltin oxide, and react at 100°C for 80 minutes to obtain polymer A;
[0057] S2, add 10 mass parts polyethylene glycol methyl ether methacrylate (molecular weight 20000), 5 mass parts polyethylene glycol methyl methacrylate (molecular weight 300), 10 mass parts polyethylene oxide (molecular weight 10 million) In 200 parts by mass of tetrahydrofuran, continue nitrogen flow, stir at a speed of 800r / min, then add 5 parts by mass of lithium bisoxalate borate (LiBOB), 0.05 parts by mass of benzoyl tert-butyl peroxide, and then add and mix 5 parts by mass of Methoxy polyethylene glycol borate and 5 parts by mass of nano-silica (700nm particle size) of toluene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com