DNA nucleic acid constant value quality control material and preparation method thereof
A quality control material, HIV-2DNA technology, applied in the field of medical testing, can solve problems such as difficulty in ensuring the validity and consistency of measurement results, differences in the accuracy and traceability of quantitative values, and delays in the development of related quality control materials, and achieve stability Good stability and uniformity, accurate target value determination, and the effect of ensuring accuracy and reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
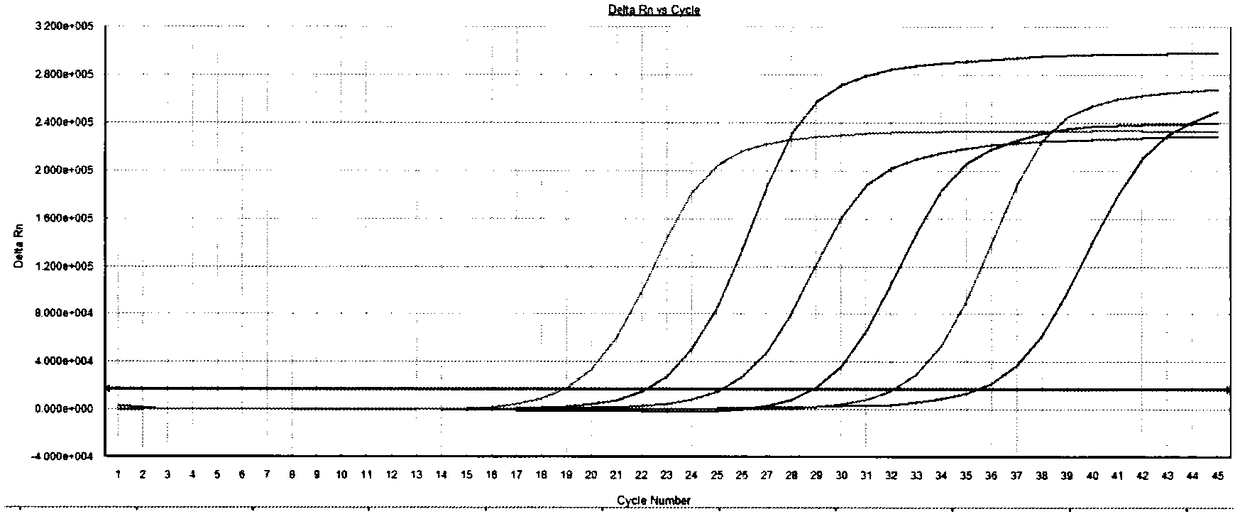
[0036] Example 1-HIV-2 DNA droplet digital PCR method establishment
[0037] (1) Design of primers and probes:
[0038] According to the relevant information of HIV-2 gene sequence, two pairs of PCR primer probes were designed with the aid of Primer Premier5.0 biological software.
[0039] Table 1 Primers and probes for PCR amplification of HIV-2 gag-gp105 genome
[0040]
[0041]
[0042] (2) Extraction of viral nucleic acid (Tiangen Magnetic Bead Method Viral DNA / RNA Extraction Kit)
[0043] a. Add 20μl proteinase K to a clean 1.5ml centrifuge tube.
[0044] b. Add 200μl of plasma / serum / lymph to the centrifuge tube (the sample needs to be equilibrated to room temperature).
[0045] c. Add 400μl Carrier working solution to the sample, close the cap, and vortex for 10 seconds to mix. d. Incubate at 56°C or room temperature for 10 minutes. Centrifuge briefly to collect the liquid adhering to the tube wall and tube cap.
[0046] e. Add 400μl of absolute ethanol, flocculent precipitation ma...
Embodiment 2
[0073] Example 2-Preparation of HIV-2 standards and establishment of QPCR method
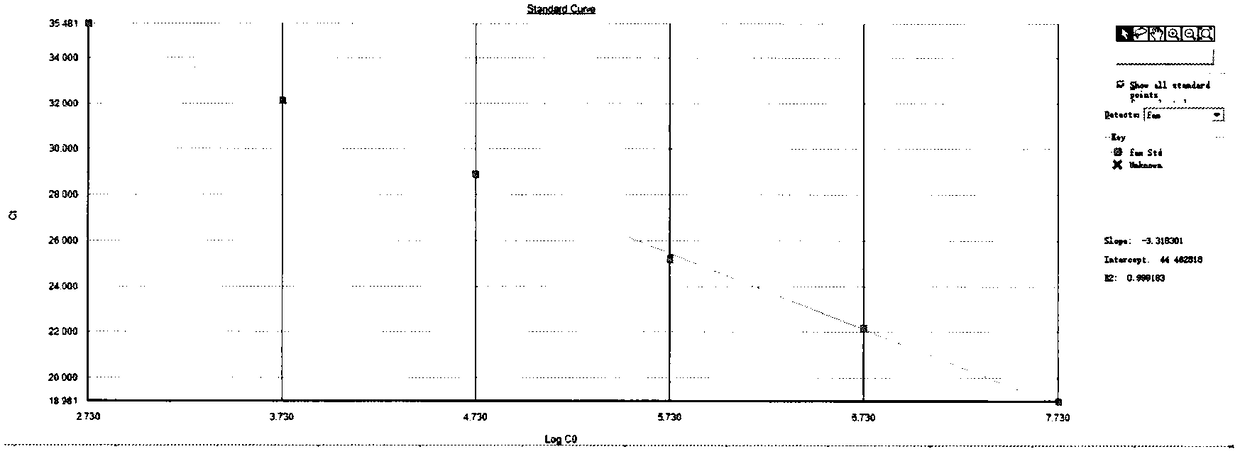
[0074] Take HIV-2 viral nucleic acid as a template and use full-length primers for amplification. The amplified product was ligated to the pEASY-T3 vector, and then transformed into Trans T1 competent cells. Bacteria containing positive recombinant plasmids are screened out and sent to sequencing for identification. Then, using the purified SPE I digestion linearized product as a template, the NanoDrop2000 ultraviolet spectrometer was used to determine the concentration, and the copy number was calculated, and a 10-fold dilution was performed. Calculate the number of DNA copies per microliter volume according to the following formula. y(copies / μL)=[x (g / μL)DNA / (transcript length in nucleotides×340)]×6.02×10 23
[0075] Using the designed primers and probes to establish the QPCR method, the QPCR reaction system is ProbeqPCR dUTP Master Mix 10μL, 10μM primer 0.8μL, 10μM probe 0.5μL, water 5.9μL, te...
Embodiment 3
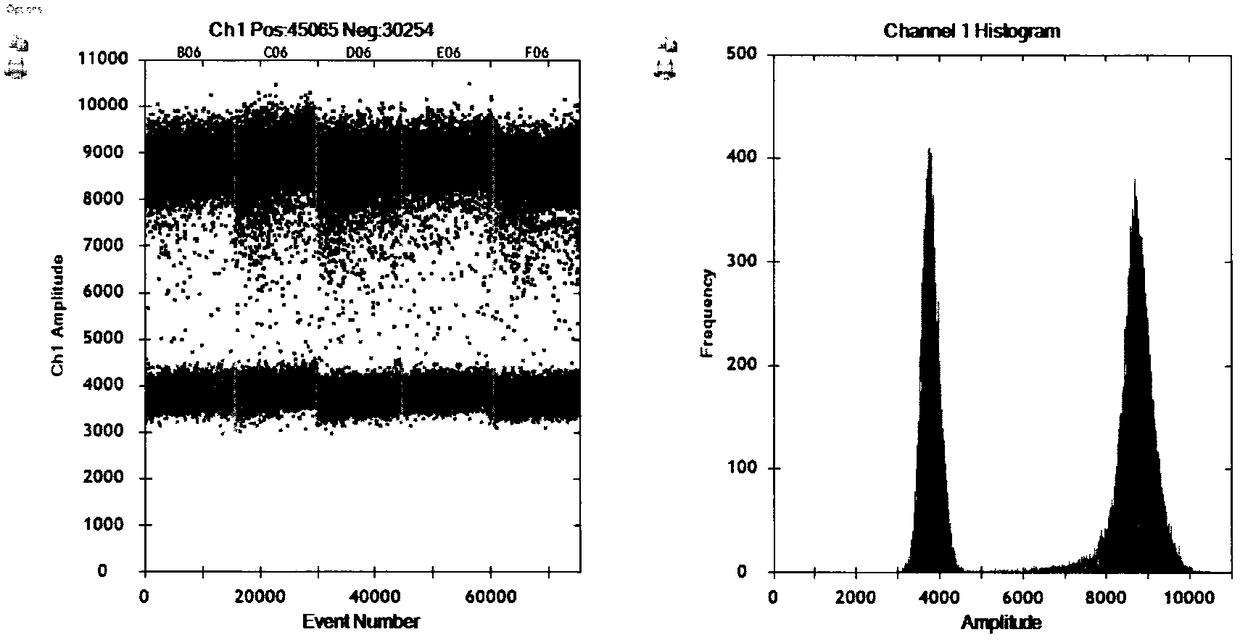
[0081] Example 3-Preparation of negative and positive samples of DNA-type nucleic acid quality control materials
[0082] (1) Negative plasma (serum) sample
[0083] The appearance properties are screened out and the appearance package is complete, without damage and leakage. There is a clear label, indicating the blood supply unit, blood donor information, preparation time, expiration date, volume, storage conditions, etc. After thawing, it should be a light-yellow homogeneous liquid, without obvious flocculent sediments or large pieces of sediment, and the result of the contamination test (PCR method) is negative.
[0084] After the qualified negative plasma is inactivated at 56°C for 60 minutes, fibrin is removed by filtration with sterile medical gauze in a Class II biological safety cabinet in a Class 10,000 work area, and stored at -20±5°C for use.
[0085] (2) Positive plasma (serum) sample
[0086] The samples were tested using the nucleic acid extraction method of Example 2 a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com