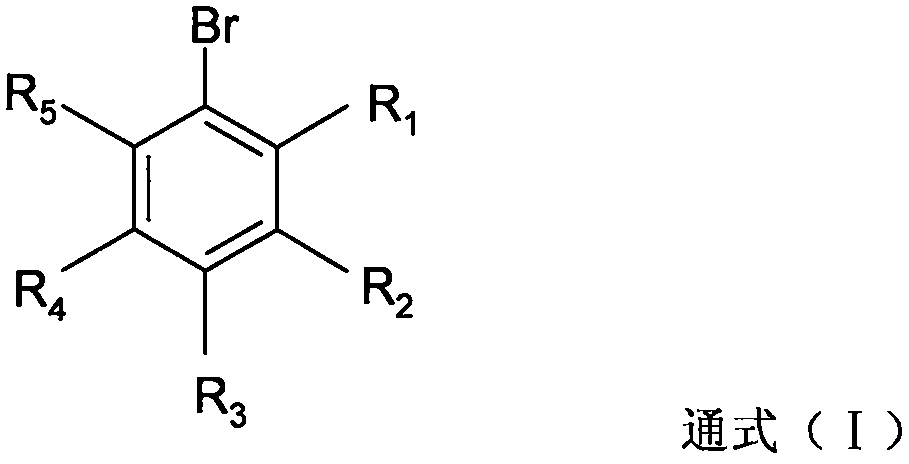
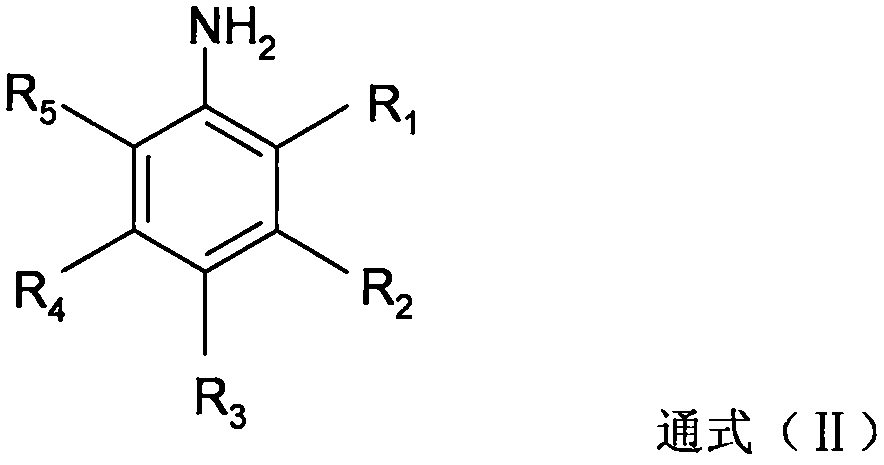
Novel process for synthesizing high-steric-hindrance bromobenzene
A bromobenzene, a new process technology, applied in the new process field of bromobenzene, can solve the problems of difficult rectification separation, difficult to improve purity, waste water generation, etc., to achieve reduced yield reduction, high selectivity, and production safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
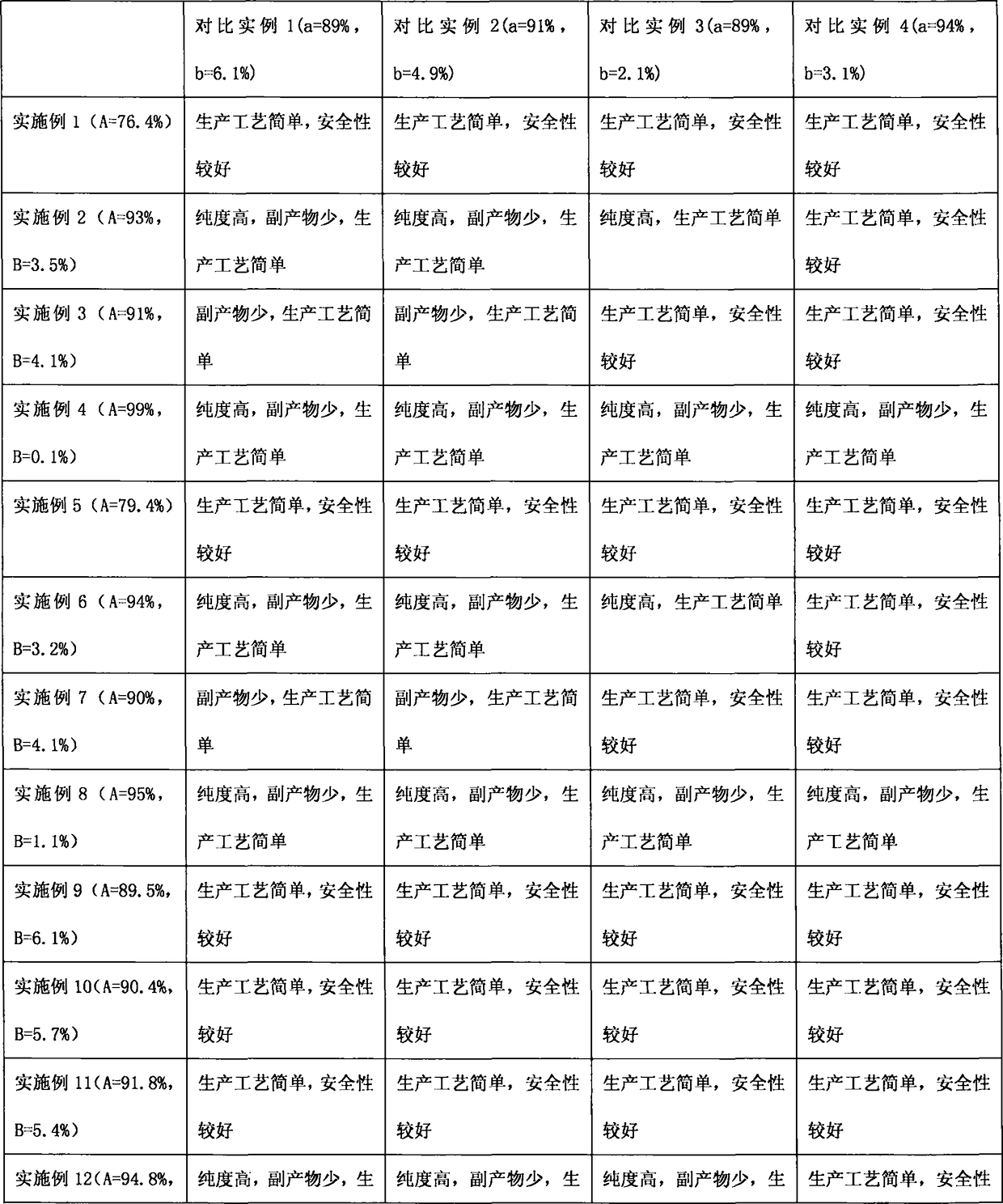
Embodiment 1
[0036] In 0.250 moles of 2,6-diethyl-4-methylaniline, 0.125 moles of phosphoric acid and 0.125 moles of 3-picoline in 150 g of 2-methyltetrahydrofuran solution, add 72.2 g of 42% hydrobromic acid . Heat the resulting mixed solution up to 80°C, the water separator starts to reflux to divide the water, then cool down to 55-62°C, add 0.326 moles of solid sodium nitrite and 0.172 moles of 42% hydrobromic acid in batches, and react after completion The temperature of the mixture rose to 70-75°C. Then add 0.6g of urea, separate the organic phase, and evaporate the solvent from the organic phase to obtain 62.2g of reddish-brown 1-bromo-2,6-diethyl-4-methylbenzene crude product, which is obtained by rectifying the crude product under reduced pressure 49.9 g of yellow 1-bromo-2,6-diethyl-4-methylbenzene oil, the gas phase detection purity is 76.4%.
Embodiment 2
[0038] Pass 3.15 moles of hydrogen bromide gas into the solution of 3.00 moles of 2,6-diethyl-4-methylaniline and 1050 g of chlorobenzene to obtain 2,6-diethyl-4-methylaniline hydrogen bromide suspension, and then added 0.20 moles of trichloroacetic acid and 9.6 g of 42% hydrobromic acid. Heat the reaction mixture to 49-55°C, add 1.00 mole of sulfuric acid and 1.30 mole of sodium nitrite in batches, and remove water azeotropically under reduced pressure after completion. Then, at this temperature, 0.68 moles of sulfuric acid and 1.90 moles of sodium nitrite were added in batches again, until tracking detection showed that the raw material 2,6-diethyl-4-methylaniline was less than 0.1%. After the organic phase was washed twice with water, chlorobenzene was evaporated under reduced pressure, and then rectified under reduced pressure to obtain 630.2 g of yellow 1-bromo-2,6-diethyl-4-methylbenzene oil, which was analyzed by gas chromatography. , with a purity of 93%, containing a...
Embodiment 3
[0040]3.00 moles of 2,6-diethyl-4-methylaniline were dissolved in 1050 g of chlorobenzene, then 3.00 moles of 42% hydrobromic acid were added, and then decompression azeotropic water removal gave 2,6-diethyl Base-4-methylaniline hydrogen bromide suspension, then added 0.25 moles of trichloroacetic acid and 10.3 g of 42% hydrobromic acid. Heat the reaction mixture to 49-55°C, add 1.00 mole of sulfuric acid and 1.50 mole of sodium nitrite in batches, and remove water azeotropically under reduced pressure after completion. Then, at this temperature, 0.62 moles of sulfuric acid and 1.76 moles of sodium nitrite were added in batches again until tracking detection showed that the raw material 2,6-diethyl-4-methylaniline was less than 0.1%. After the organic phase was washed twice with water, chlorobenzene was evaporated under reduced pressure, and then rectified under reduced pressure to obtain 585.4 g of yellow 1-bromo-2,6-diethyl-4-methylbenzene oil, which was analyzed by gas chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com