Beta-agarase with homogeneous degradation products and application thereof
A technology of degradation products and agarase, which is applied in the biological field, can solve problems such as complex components, and achieve the effects of uniform product, good industrial application prospects, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
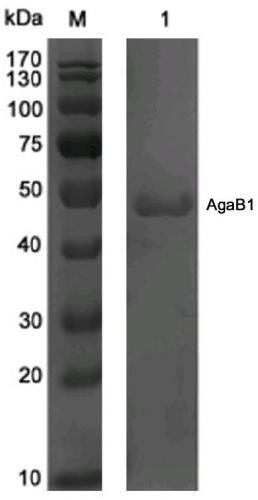
[0026] Example 1 Artificial design and sequence analysis of β-agarase AgaB1
[0027] β-agarase gene of the present invention agaB1 Artificially designed, fully synthetic sequence (synthesized by Huada Gene Company), including 1,620 base sequences, encoding 540 amino acid sequences, the N-terminal is the catalytic region (R1-V392), and the C-terminal contains a 148-amino acid sequence Carbohydrate binding domain (G393-Y540), this domain region can enhance the substrate recognition ability of β-agarase enzyme and change the distribution of degradation products. Conserved domain (CDD, https: / / www.ncbi.nlm.nih.gov / cdd) was analyzed using the conserved domain in National Center for Biotechnology Information (NCBI, https: / / www.ncbi.nlm.nih.gov / ) and Multiple Sequence Alignment Basic Local Alignment SearchTool (Blast, https: / / blast.ncbi.nlm.nih.gov / ) found that the C-terminus of the sequence contained a β-agarase polysaccharide hydrolase family 50 (GH family 50), the N-terminus c...
Embodiment 2
[0028] Example 2 Gene cloning and recombinant expression of β-agarase AgaB1
[0029] Fully synthesized in Example 1 agaB1 restriction endonuclease Nco I and xho I (purchased from Dalian Bao Biological Co., Ltd.) is the enzyme cutting site and the protection base of the enzyme cutting site is designed, and the recombinant primers are designed as follows (the underline is the restriction endonuclease site, and the italic is the restriction endonuclease protection base) :
[0030] Forward primer: SEQ ID 3: PAgaB1EF:
[0031] 5'- CATG CCATGG GTCGTGTAGATGCAAAAAA -3' ( Nco I)
[0032] Reverse primer: SEQ ID 4: PAgaB1ER:
[0033] 5'- CCG CTCGAG GTAAATGTTCCATTCCCAAAA-3’ ( xho I)
[0034] The β-agarase gene was amplified by PCR using the above-mentioned recombinant primers, and the high-fidelity DNA polymerase PrimerstarHS used in PCR was purchased from Dalian Bao Biology Company. The specific PCR amplification conditions are: pre-denaturation at 94°C for 3 mi...
Embodiment 3
[0037] Example 3 Fermentation process and purification preparation method of β-agarase AgaB1
[0038] The Escherichia coli BL21(DE3) / pET22b-AgaB1 constructed in Example 2 and stored at -80°C was streaked on the LB solid plate, and after culturing at 37°C for 16 hours, single clones were picked; 50 μg / mL ampicillin in LB liquid medium (500 mL Erlenmeyer flask loaded with 50 mL liquid medium), cultured in a shaker at 37°C at 180 rpm to OD 600 =0.6. The 5 L fermenter was loaded with 60% (3 L) Terrific Broth (TB) medium, and sterilized in advance; 50 μg / mL ampicillin was added to the fermenter, and the cultured bacteria in the Erlenmeyer flask The solution was inoculated into a 5 L fermenter according to the inoculum amount of 2%. Adjust the initial ventilation rate to 50 L / h, the initial rotation speed to 350 rpm, the temperature at 37°C, and the dissolved oxygen at 15-40%; when the bacteria grow to OD 600 When =5.0, add the inducer isopropyl-β-D-thiogalactopyranoside (IPTG) at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com