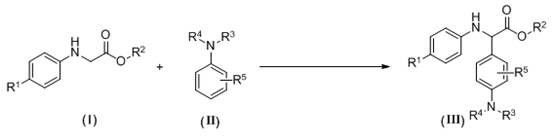
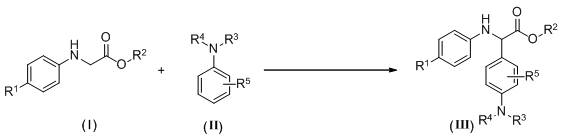
A kind of synthetic method of α-aryl substituted glycine ester derivatives
A synthesis method and technology of glycinate, applied in the direction of chemical instruments and methods, preparation of organic compounds, cyanide reaction preparation, etc., can solve the problems of large amount of oxidant, metal residues, application restrictions, etc., and achieve high product yield and easy operation Convenience and good reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 2-(p-Toluidine) ethyl acetate (0.5 mmol, 96.6 mg), N - Add methylaniline (1.0 mmol, 107 mg) and methylene blue (0.025 mmol, 8 mg) into a 25 mL two-necked flask, then add tetrahydrofuran (5 mL) as a solvent, stir, and irradiate with blue light from a 3W LED lamp , reacted at a temperature of 40°C in an air atmosphere for 8 hours. After the reaction was completed, water and methylene chloride were added to the reaction system, and the layers were extracted and separated into an aqueous layer and an organic layer. Anhydrous sodium sulfate was added to the organic layer for drying. The dried organic layer was concentrated by distillation under reduced pressure to obtain a yellow oil, which was separated and purified by column chromatography on silica gel, using a mixture of petroleum ether and ethyl acetate at a volume ratio of 20:1 as an eluent, and collected The eluent was evaporated to remove the solvent to obtain 127 mg of yellow oily product with a yield of 85%, which ...
Embodiment 2
[0026] 2-(p-Toluidine) tert-butyl acetate (0.5 mmol, 110.6 mg), N - Add methylaniline (0.6 mmol, 64.2 mg) and methylene blue (0.025 mmol, 8 mg) into a 25 mL two-necked flask, then add acetonitrile (5 mL) as solvent, stir, and irradiate with blue light from a 3W LED lamp Under air atmosphere, react at a temperature of 50°C for 12h. After the reaction, add water and methylene chloride to the reaction system, extract and separate layers, and divide into an aqueous layer and an organic layer. Add anhydrous sodium sulfate to the organic layer for drying , the dried organic layer was concentrated by distillation under reduced pressure to obtain a yellow oil, and the obtained yellow oil was separated and purified by column chromatography on silica gel, and a mixed solution of petroleum ether and ethyl acetate with a volume ratio of 40:1 was used as an eluent. Collect the eluate and evaporate the solvent to obtain 122 mg of yellow solid product with a yield of 75%, namely the target p...
Embodiment 3
[0029] 2-(p-Cymeniline) ethyl acetate (0.5 mmol, 110.6 mg), N - Methylaniline (0.8 mmol, 85.6 mg), methylene blue (0.05 mmol, 16 mg) was added to a 25 mL two-necked flask, and then 1,2-dichloroethane (5 mL) was added as a solvent, stirred, and at a power of 3W Under the blue light from the LED lamp, react at 50°C for 16 hours in the air atmosphere. After the reaction,
[0030] Add water and dichloromethane to the reaction system, extract and separate layers, divide into water layer and organic layer, add anhydrous sodium sulfate to the organic layer to dry, and the dried organic layer is concentrated by vacuum distillation to obtain a yellow oil, and the obtained yellow The oil was separated and purified by column chromatography on silica gel, using a mixture of petroleum ether and ethyl acetate at a volume ratio of 20:1 as the eluent, collecting the eluent and evaporating the solvent to obtain 102.7mg of a yellow oily product, the yield Be 63%, obtain target product, its str...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com