High-yield production process for 1,3-diphenyl propanedione compound
A technology for the production of diphenylpropanedione, which is applied in the separation/purification of carbonyl compounds, the preparation of carbon-based compounds, and the preparation of organic compounds. problems, to achieve the effect of strong market competitiveness, reduction of by-products and three wastes, and reduction of side reactions and impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
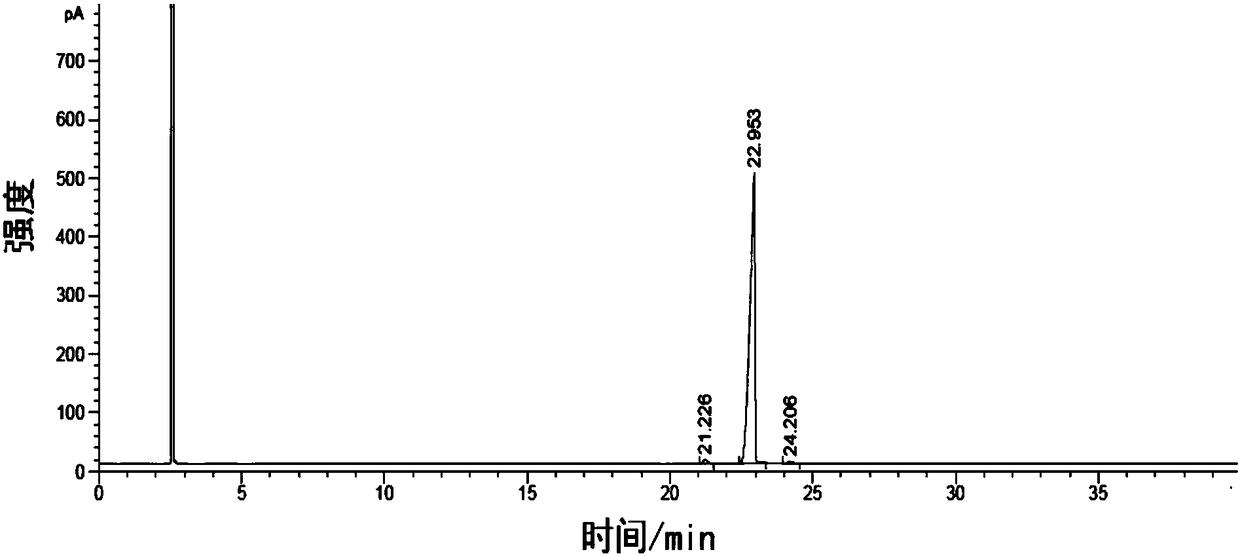
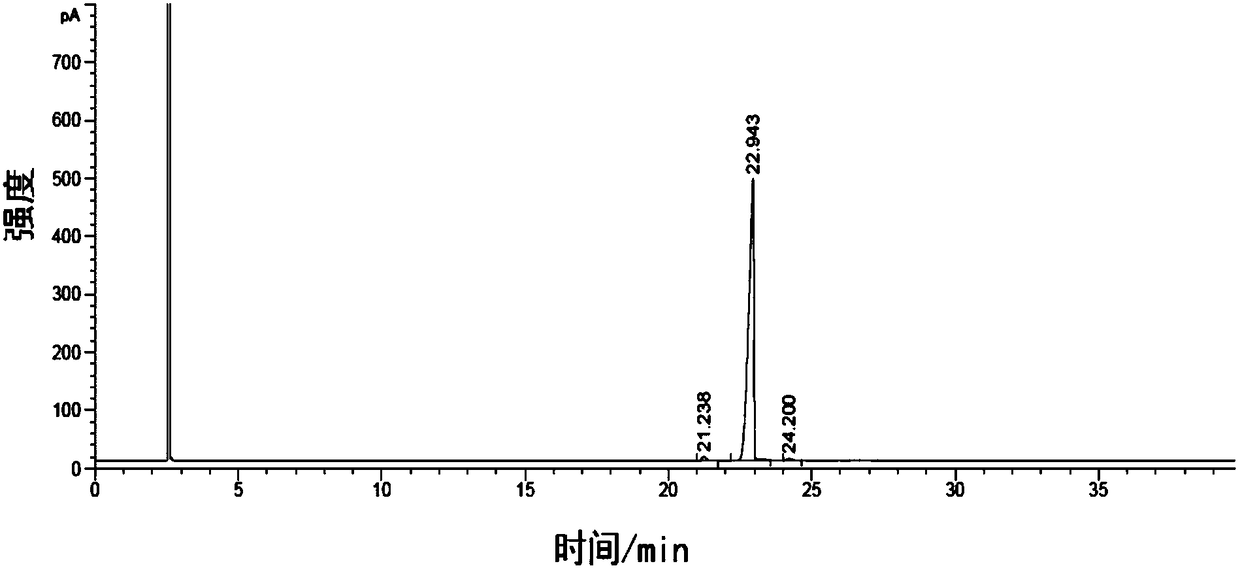
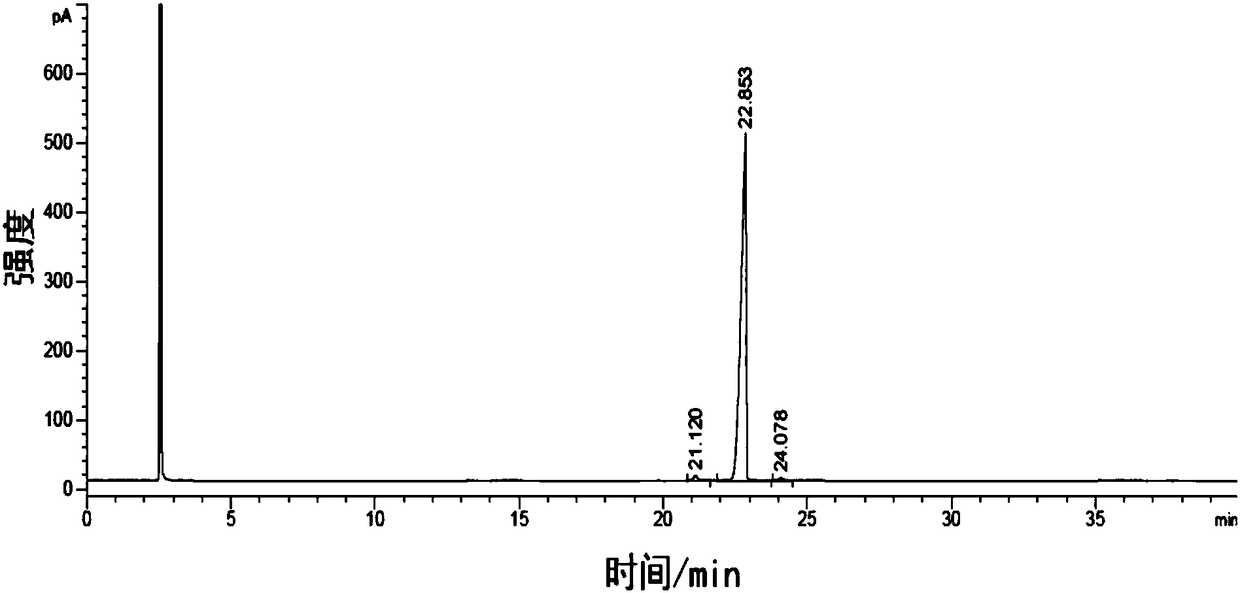
Image
Examples
Embodiment 1
[0086] Add 800g toluene (toluene), 24g sodium methylate, 6g 32% liquid potassium methylate solution and 80g p-tert-butylbenzoic acid methylate Esters, fractionate methanol while raising the temperature until the temperature of the kettle reaches 105-108°C;
[0087] Start to drop the mixed solution composed of 60g p-methoxyacetophenone and 80g toluene, and at the same time fractionate the methanol produced by reaction from the top of the rectifying column to keep the temperature of the kettle at 105-108°C, and the dropping time is controlled at 1.5h. After the dropwise addition is completed, keep the reaction for 2 hours. During the heat preservation process, the methanol by-product of the reaction is continuously fractionated from the top of the rectification column to maintain the temperature of the kettle at 105-108°C.
[0088] After the reaction was finished, add 120 g of 10 (weight) % dilute sulfuric acid aqueous solution, stir and wash and then separate the water layer, ...
Embodiment 2
[0092] Add 800g of toluene, 12g of sodium methoxide, and 80g of methyl p-tert-butylbenzoate in a reaction kettle with a rectification column (stacked θ-ring stainless steel filler, 20 theoretical plates), fractionate methanol while raising the temperature, and reach the temperature of the kettle. Reach 105~108℃;
[0093] Start to drop the mixed solution composed of 60g p-methoxyacetophenone and 80g toluene, and at the same time fractionate the methanol produced by reaction from the top of the rectifying column to keep the temperature of the kettle at 105-108°C, and the dropping time is controlled at After 1.5h, 6g32% liquid potassium methylate solution was added dropwise for 0.5h. After the dropwise addition is completed, add 12 g of sodium methoxide again, and keep the reaction for 2 hours. During the heat preservation process, the methanol by-product of the reaction is continuously fractionated from the top of the rectification column to maintain the temperature of the kettl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com