High-sensitivity iron ion probe and application thereof
A high-sensitivity, iron-ion technology, applied in the field of fluorescent probes, can solve problems such as being unsuitable for mass detection and real-time detection, and achieve the effects of high yield, short time, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
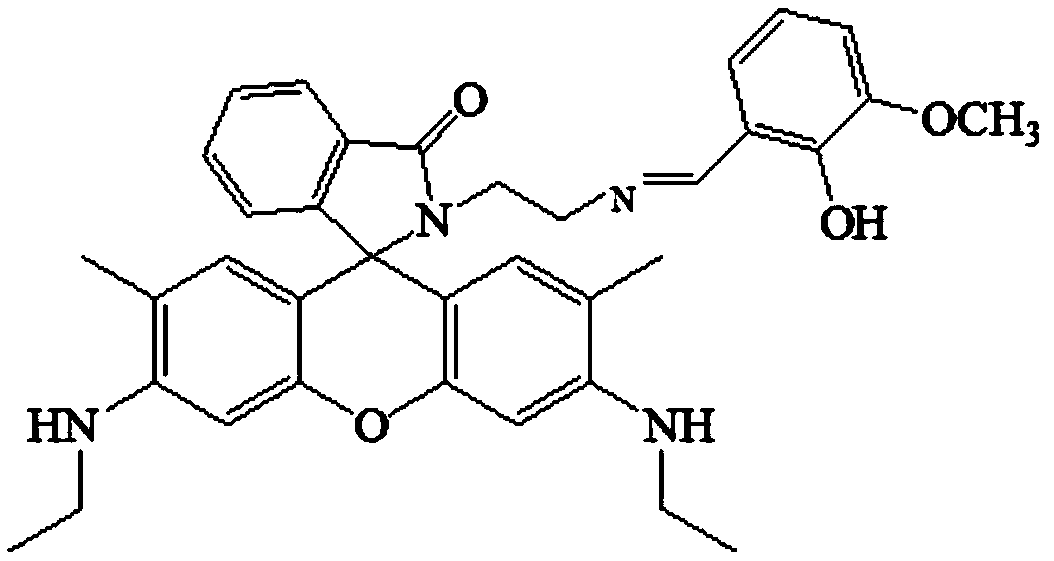
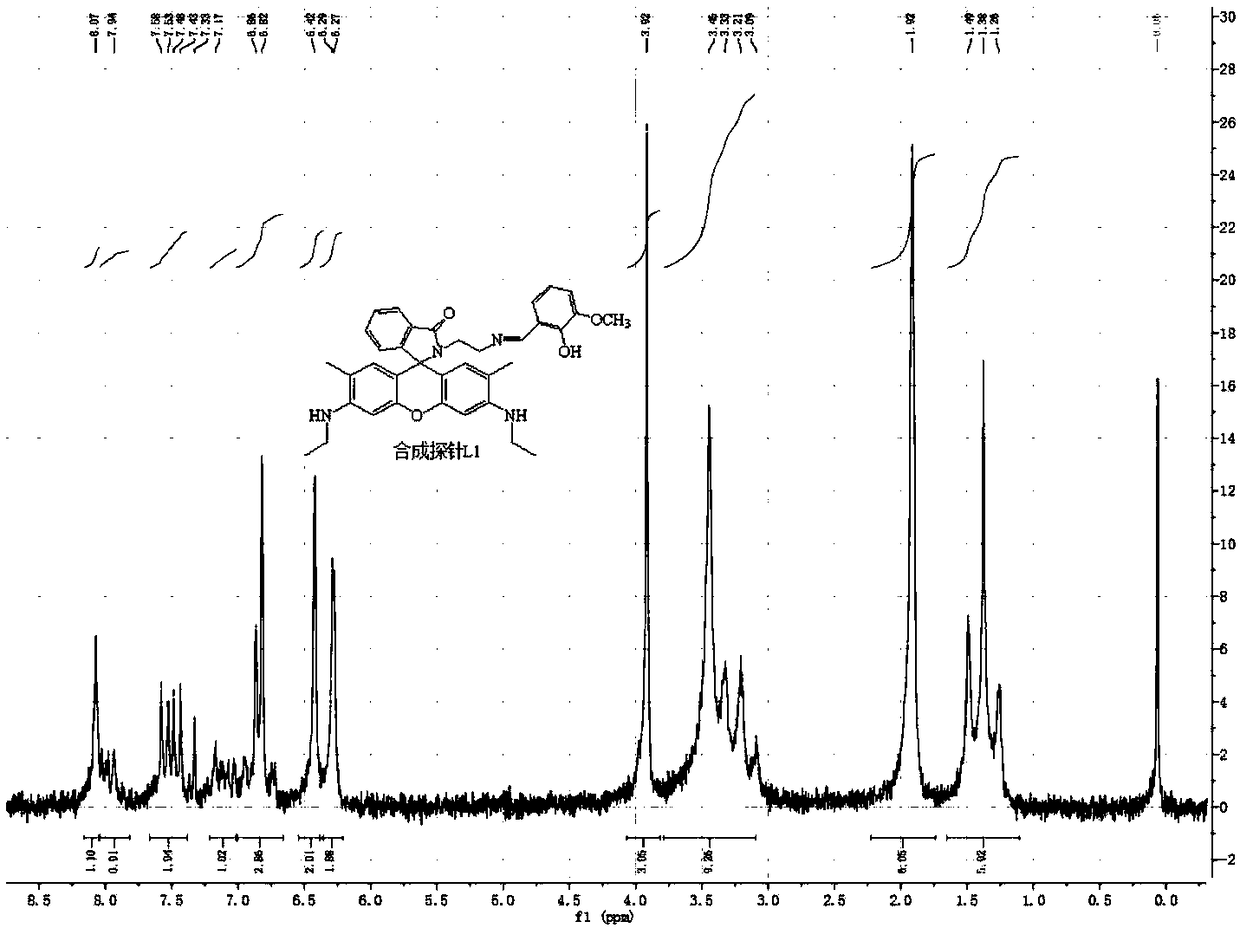
[0037] The preparation of embodiment 1 iron ion probe
[0038] (1) Synthesis of Rhodamine 6G:
[0039] a, 5.0000g 3-ethylamino-4-methylphenol, 15.0000g phthalic anhydride and 4.8000g zinc chloride are placed in a mortar and ground, and poured into an eggplant-shaped flask after grinding evenly (the eggplant-shaped flask The size is selected according to the needs of use), heat the flask, keep the temperature at 165 ° C, and slowly stir the reaction for 1 hour, so that the mixture gradually melts and the color becomes darker. At this time, careful stirring is required. As the reaction progresses, the consistency gradually It becomes smaller, and then gradually becomes larger, and the reactant presents a metallic brown-purple color; then decompress and vacuumize, and remove the cork after 5 to 10 minutes until no phthalic anhydride sublimates in the bottle;
[0040] b. Dissolve the mixture obtained in step a in 120mL of N,N-dimethylformamide (DMF), then slowly drop into 1200mL ...
Embodiment 2
[0054] Ion response and selectivity of the iron ion probe of embodiment 2
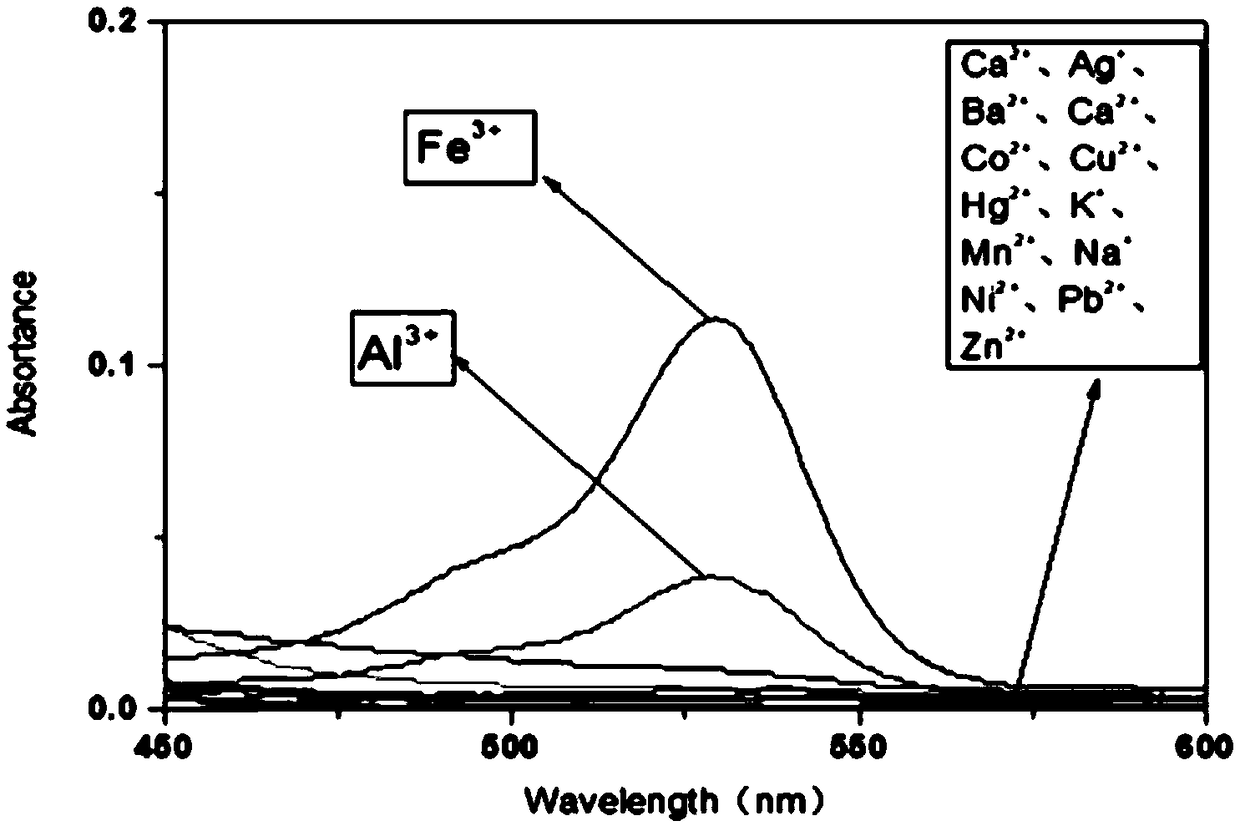
[0055] Add 2940 μL of acetonitrile aqueous solution (the volume ratio of water and acetonitrile is 1:4) and 30 μL of iron ion probe solution (weigh 88 mg of the target product prepared in Example 1 to 50 mL) into 15 7 mL centrifuge tubes with a pipette gun. In a volumetric flask, dissolve with acetonitrile and make up to volume with acetonitrile), then add 60 μL of prepared 3mmol / L metal ion solution to 14 centrifuge tubes, each metal ion solution corresponds to a centrifuge tube, one of which does not contain The acetonitrile aqueous solution of the probe solution was used as a blank sample, and was labeled with label paper; the 14 metal ions were Ag + 、Al 3+ 、Ba 2+ , Ca 2+ 、Co 2+ 、Cu 2+ , Fe 3+ , Hg + 、K + , Mn + 、Na + 、Ni + , Pb + , Zn 2+ .
[0056] When adding Fe 3+ solution, it was found that the color of the solution in the centrifuge tube changed from light yellow to colorless, an...
Embodiment 3
[0060] Interference of embodiment 3 iron ion probe
[0061] In this example, Fe 3+ Whether it interferes with the response of other metal ions to detect ion interference, use a pipette gun to add 60 μL of Fe 3+ solution, and shake well. Join Fe 3+ After the solution, the colors of 14 centrifuge tubes all turned red, indicating that other metal ion solutions had no effect on the iron ion probe of the present invention.
[0062] Then detect the ultraviolet absorption situation of the solution in these 14 centrifuge tubes with the ultraviolet spectrophotometer, the wavelength is set at 300~600nm, the result is as follows Figure 4 shown. The results show that when adding Fe 3+ After solution, the solutions in all 14 centrifuge tubes showed obvious ultraviolet absorption peaks at 530nm, and the absorbance changed. Among them, Fe 3+ The absorbance is significantly higher than that of other metal ions and Fe 3+ The absorbance of other metal ions on Fe 3+ There is no interfer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com