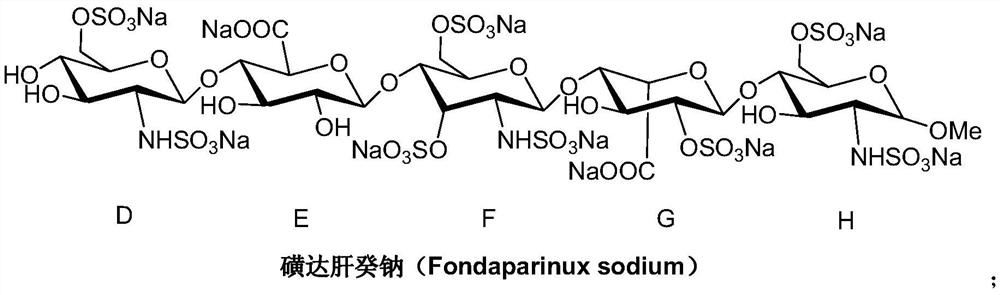
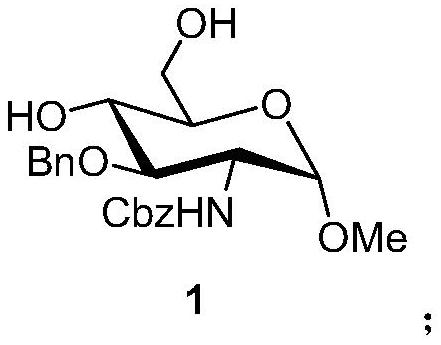
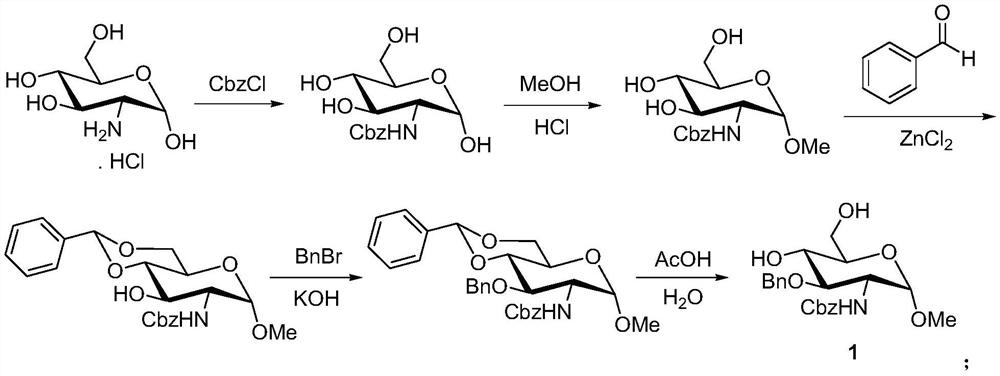
A kind of preparation method of fondaparinux sodium monosaccharide intermediate
A technology of reaction time and glucopyranoside, which is applied in the field of preparation of fondaparinux sodium monosaccharide intermediates, can solve the problems of many side reactions, affecting product quality, purity and yield, etc., so as to reduce side reactions and cost Effect of reducing, simplifying and optimizing reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A) Preparation of methyl 4,6-O-benzylidene-2-amino-2-deoxy-α-D-glucopyranoside (compound shown in formula (4)):
[0048] 4,6-O-benzylidene-2-glucosamine (25g, the compound represented by formula (5)) was mixed with methanol (75mL) and dissolved, and hydrochloric acid (300mL, mass fraction was 30%) was added, and the temperature was raised to 60°C React for 36 hours until the reaction is complete, lower to room temperature, adjust the pH value to 8-9 with potassium carbonate solution, cool to 0°C for crystallization for 6 hours, filter with suction, and recrystallize the filter cake with methanol to obtain methyl 4,6-O -Benzylidene-2-amino-2-deoxy-α-D-glucopyranoside, white solid 23.7g, yield 90%, purity 98.5%.
[0049] B) Preparation of methyl 4,6-O-benzylidene-2-(benzyloxycarbonyl)amino-2-deoxy-α-D-glucopyranoside (compound represented by formula (3)):
[0050] Dissolve methyl 4,6-O-benzylidene-2-amino-2-deoxy-α-D-glucopyranoside (22.5g) in chloroform (300mL), add wat...
Embodiment 2
[0056] A) Preparation of methyl 4,6-O-benzylidene-2-amino-2-deoxy-α-D-glucopyranoside (compound shown in formula (4)):
[0057] 4,6-O-benzylidene-2-glucosamine (44g, the compound represented by formula (5)) was mixed with methanol (7.9g), heated to dissolve, added concentrated sulfuric acid (8.1g), raised to 55°C for reaction After 48 hours until the reaction is complete, cool down to room temperature, adjust the pH value to 8-9 with potassium carbonate solution, cool to 0°C and crystallize for 6 hours, filter with suction, and recrystallize the filter cake with methanol to obtain methyl 4,6-O- Benzylidene-2-amino-2-deoxy-α-D-glucopyranoside, white solid 39.8g, yield 86%, purity 97.7%.
[0058] B) Preparation of methyl 4,6-O-benzylidene-2-(benzyloxycarbonyl)amino-2-deoxy-α-D-glucopyranoside (compound represented by formula (3)):
[0059] Methyl 4,6-O-benzylidene-2-amino-2-deoxy-α-D-glucopyranoside (35 g) was dissolved in acetone (500 mL), and an aqueous solution of sodium car...
Embodiment 3
[0065] A) Preparation of methyl 4,6-O-benzylidene-2-amino-2-deoxy-α-D-glucopyranoside (compound shown in formula (4)):
[0066] 4,6-O-benzylidene-2-glucosamine (96g, the compound represented by formula (5)) and methanol (575.4g) were mixed and dissolved, and acetic acid (215.7g) was added, raised to 65°C for 24h to react complete, lowered to room temperature, adjusted to pH 8-9 with potassium carbonate solution, cooled to 0°C for crystallization for 6 hours, filtered with suction, and the filter cake was recrystallized with methanol to obtain methyl 4,6-O-benzylidene- 2-Amino-2-deoxy-α-D-glucopyranoside, white solid 89.9g, yield 89%, purity 97.9%.
[0067] B) Preparation of methyl 4,6-O-benzylidene-2-(benzyloxycarbonyl)amino-2-deoxy-α-D-glucopyranoside (compound represented by formula (3)):
[0068] Methyl 4,6-O-benzylidene-2-amino-2-deoxy-α-D-glucopyranoside (88g) was dissolved in 1.2-dichloroethane (1L), potassium bicarbonate (78.3 g) and benzyl chloroformate (106.7g), kee...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com