Method for producing alpha-ketoisovaleric acid with high yield
A ketoisovaleric acid and amino acid technology, applied in the field of bioengineering, can solve the problems such as no application report of L-amino acid oxidase, complicated separation and purification, residual intermediate products, etc., and is conducive to large-scale industrial production and product purity. High, mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Induced expression of recombinant genetically engineered bacteria
[0037] Construction process of recombinant bacteria:
[0038] (1) Using the genome of Corynebacterium glutamicum as a template, design primers for PCR to clone the gene encoding L-amino acid oxidase derived from Corynebacterium glutamicum. The primers used are: 5' ccgctcgagcggatgaaaattg cggtaatcgg 3' as forward primer , using 5'caagcttggataatgcacgtggcggagaaactaaccaagtac 3' as a reverse primer to amplify the target gene, the nucleotide sequence of which is shown in SEQ ID NO.2.
[0039] (2) Digest the target gene and the expression vector pET-28a with restriction endonucleases XhoI and hindIII at 37°C for 2h;
[0040] (3) Ligate the target gene and plasmid pET-28a after digestion and gel recovery with T4 ligase at 16°C for 10 h;
[0041] (4) Introduce the constructed expression plasmid into E.coli BL21(DE3), and culture it on the LB plate containing kanamycin for 12h;
[0042] (5) Perfor...
Embodiment 2
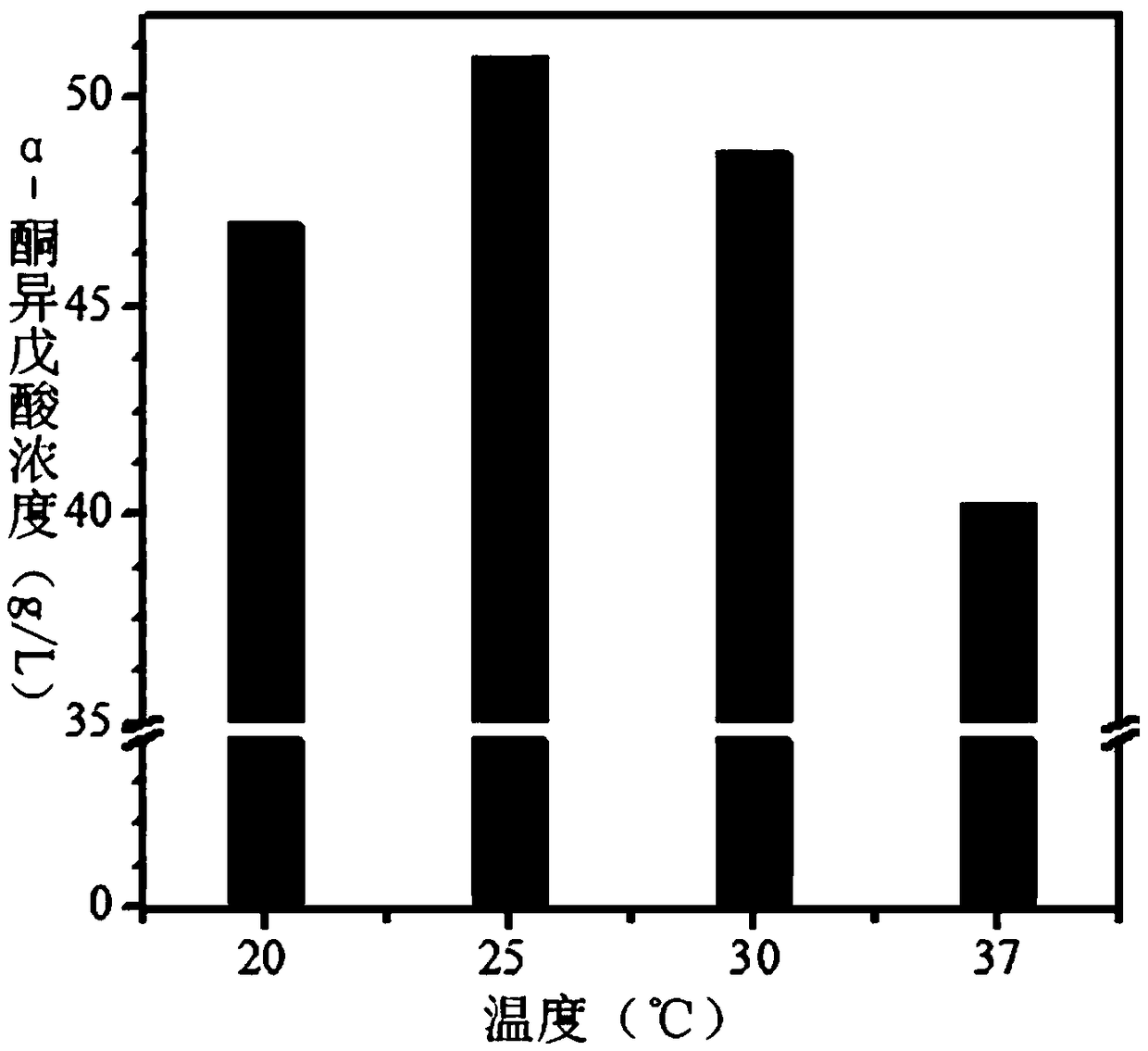
[0044] Example 2: Effect of conversion temperature on the production of α-ketoisovaleric acid
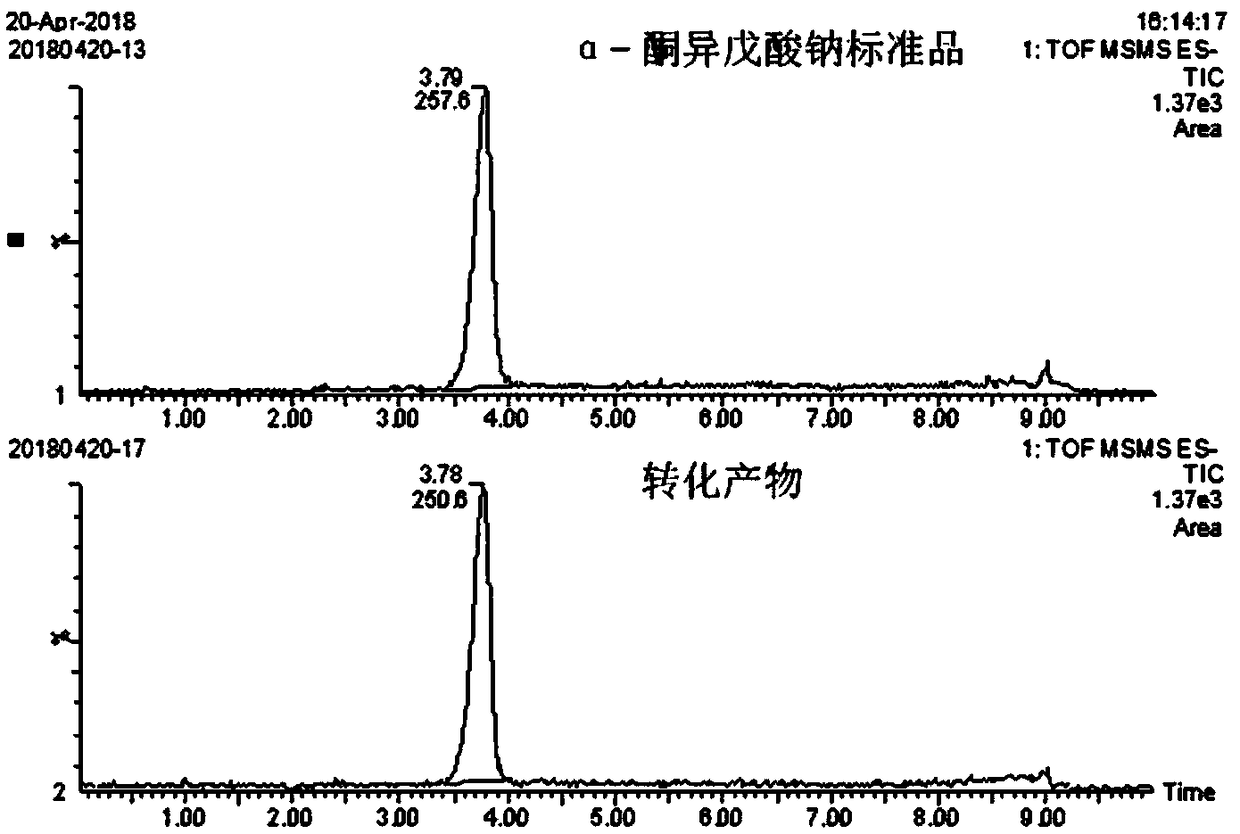
[0045] The wet thallus obtained in Example 1 was used as a catalyst for whole cell transformation. The specific transformation reaction conditions are: pH = 8.0, transformation temperature is 20°C, 25°C, 30°C, 37°C, substrate concentration is 65g / L, bacterial mass is 30g / L, transformation system is 20mL, transform at different temperatures After 24h, utilize HPLC to analyze the conversion product and the α-ketoisovalerate sodium standard substance that buys, as figure 1 As shown, it was proved that the conversion product was sodium α-ketoisovalerate. Adopt high performance liquid chromatography to measure the concentration situation of α-ketoisovaleric acid in the supernatant after the reaction (as figure 2 Shown), the result shows: when temperature is 20 ℃, ketovaline concentration is 46g / L. When the temperature is 25°C, the concentration of ketovaline is 51g / L. When the tempera...
Embodiment 3
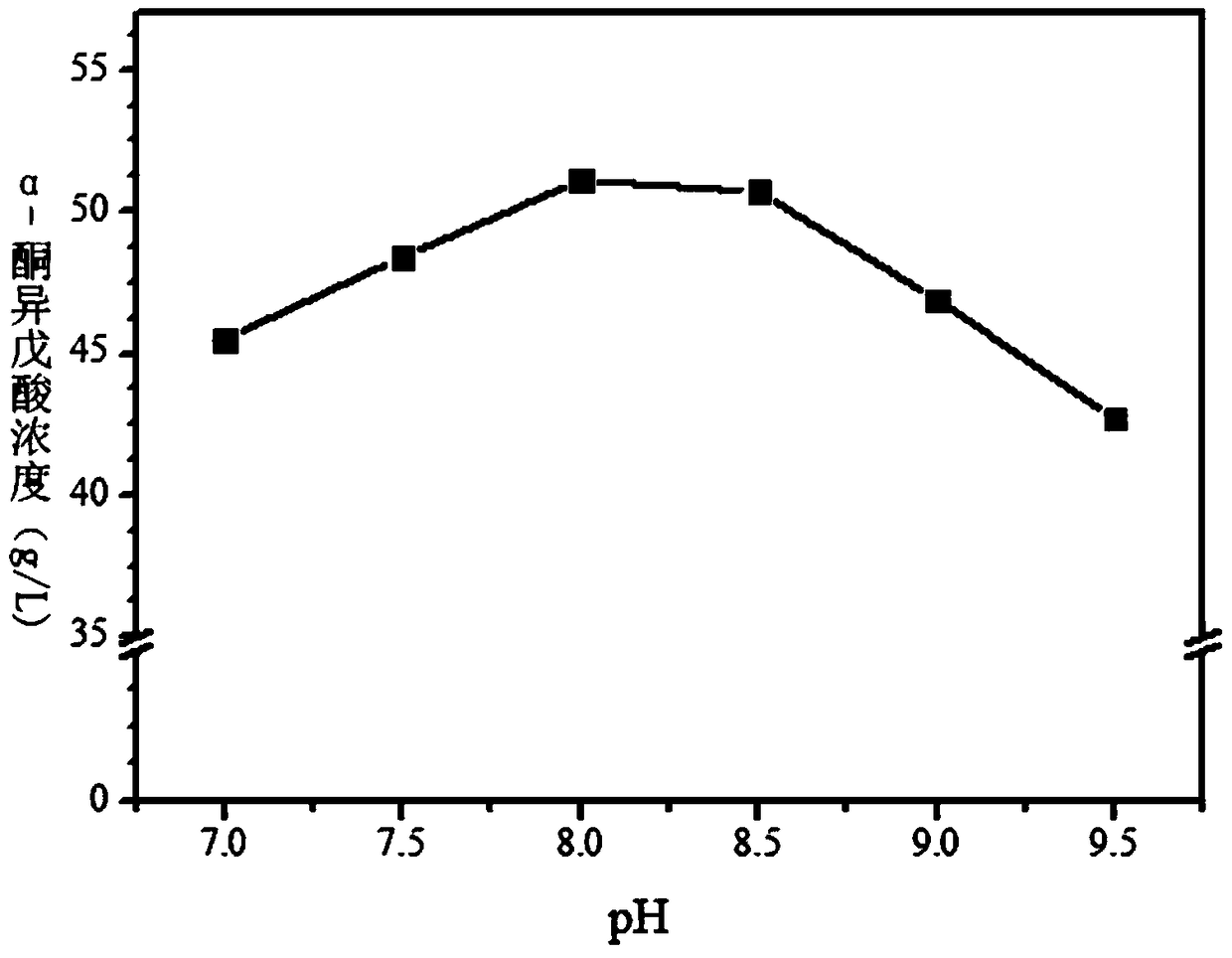
[0046] Example 3: Effect of inversion pH on the production of α-ketoisovaleric acid
[0047] The wet thallus obtained in Example 1 was used as a catalyst for whole cell transformation. The specific transformation reaction conditions are: pH=7-9.5, transformation temperature is 25°C, substrate concentration is 65g / L, bacterial mass is 30g / L, transformation system is 20mL, after transformation for 24 hours, use high performance liquid chromatography to determine The concentration of α-ketoisovaleric acid in the supernatant after the reaction (as image 3 shown), the results showed that when the pH was 7.0, the concentration of α-ketoisovalerate was 45.3g / L; when the pH was 7.5, the concentration of α-ketoisovalerate was 47.6g / L; when the pH was At 8.0, the concentration of α-ketoisovalerate is 51g / L; when the pH is 8.5, the concentration of α-ketoisovalerate is 49.4g / L; when the pH is 9.0, the concentration of α-ketoisovalerate It is 46.1g / L. When the pH is 9.5, the concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com