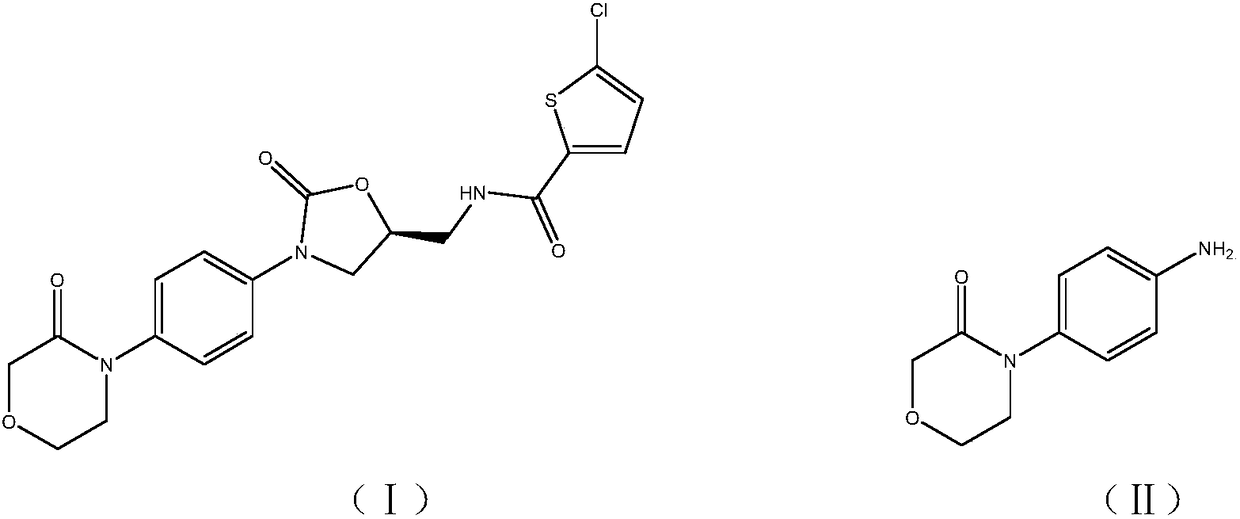
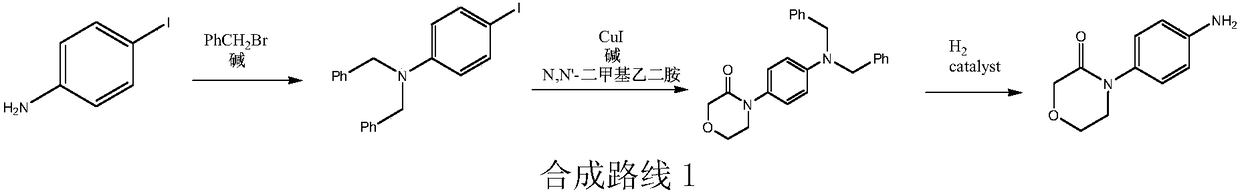
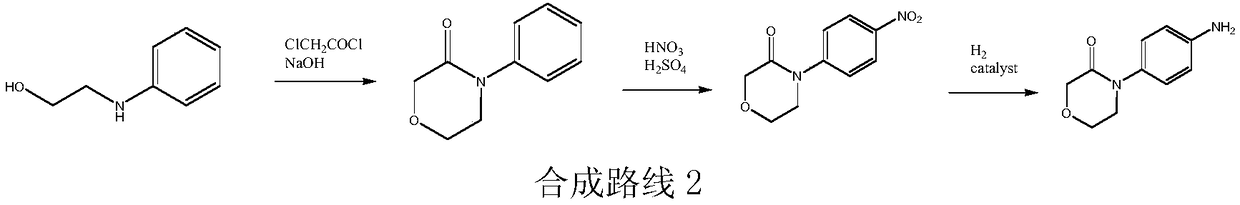
High-purity 4-(4-aminophenyl)morpholine-3-one preparation method
A technology of aminophenyl and morpholine, which is applied in the field of preparation of morpholin-3-one derivatives, can solve the problems that are not conducive to the preparation of high purity and high activity of nitrohalogenated benzene, and achieve high selectivity, simple process route, Simple effect of separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: the preparation of chloroethyloxyacetonitrile (Ⅳ1)
[0065] In a 1-liter four-necked flask connected with a stirrer, a thermometer, and a condenser tube, add 142.5 grams (1.0 moles) of hydroxyacetonitrile with a mass concentration of 40%, 300 grams of methylene chloride, 120 grams of 1,2-dichloroethane and 145 grams of potassium carbonate was heated, refluxed at 38-40°C for 5 hours, filtered, and the filter cake was washed twice with dichloromethane, 50 grams each time. Layered, the aqueous layer was extracted 3 times with dichloromethane, each with 30 grams of dichloromethane, the organic phase was combined, the organic phase was washed once with 30 grams of water, the organic phase was distilled to recover the dichloromethane, and then distilled under reduced pressure to obtain 79.2 grams Chloroethyloxyacetonitrile (Ⅳ1), yield 66.3%, GC purity 99.8%.
[0066] GC-MS analysis result (EI+, m / z): 119:121=3:1.
Embodiment 2
[0067] Embodiment 2: the preparation of chloroethyloxyacetonitrile (Ⅳ1)
[0068] In a 1-liter four-neck flask connected with a stirrer, a thermometer, and a condenser tube, add 142.5 grams (1.0 moles) of mass concentration and be 40% hydroxyacetonitrile, 300 grams of 1,2-dichloroethane and 120 grams of sodium carbonate, and heat , reacted at 60-65°C for 5 hours, filtered, and the filter cake was washed twice with 1,2-dichloroethane, 50 grams each time. Layering, the aqueous layer was extracted 3 times with 1,2-dichloroethane, each with 30 grams, the organic phase was combined, the organic phase was washed once with 30 grams of water, the organic phase was distilled to recover 1,2-dichloroethane, and then Distilled under reduced pressure to obtain 92.5 g of chloroethyloxyacetonitrile (Ⅳ1), with a yield of 77.4% and a GC purity of 99.9%.
Embodiment 3
[0069] Embodiment 3: the preparation of chloroethyloxyacetonitrile (Ⅳ1)
[0070] In a 1-liter four-necked flask connected with a stirrer, a thermometer, and a condenser tube, add 142.5 grams (1.0 moles) of mass concentration and be 40% hydroxyacetonitrile, 400 grams of methylene chloride, and 150 grams of 1-chloro-2-bromoethane And 145 grams of potassium carbonate, heated, reflux reaction at 38-40 ℃ for 4 hours, filtered, and the filter cake was washed twice with dichloromethane, each 50 grams. Separate the layers, extract the aqueous layer 3 times with dichloromethane, each time with 30 grams of dichloromethane, combine the organic phase, wash the organic phase with 30 grams of water once, distill the organic phase to recover dichloromethane and excess 1-chloro-2-bromo Ethane was then distilled under reduced pressure to obtain 103.2 g of chloroethyloxyacetonitrile (Ⅳ1), with a yield of 86.4% and a GC purity of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com