Organic polymer based on anthraquinone and preparation method and application of organic polymer as lithium-ion battery cathode material
A polymer and anthraquinone technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of limited practical application, unsatisfactory rate performance of organic electrode materials, poor conductivity, etc., and achieve excellent rate performance, cycle performance and Good magnification performance and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-
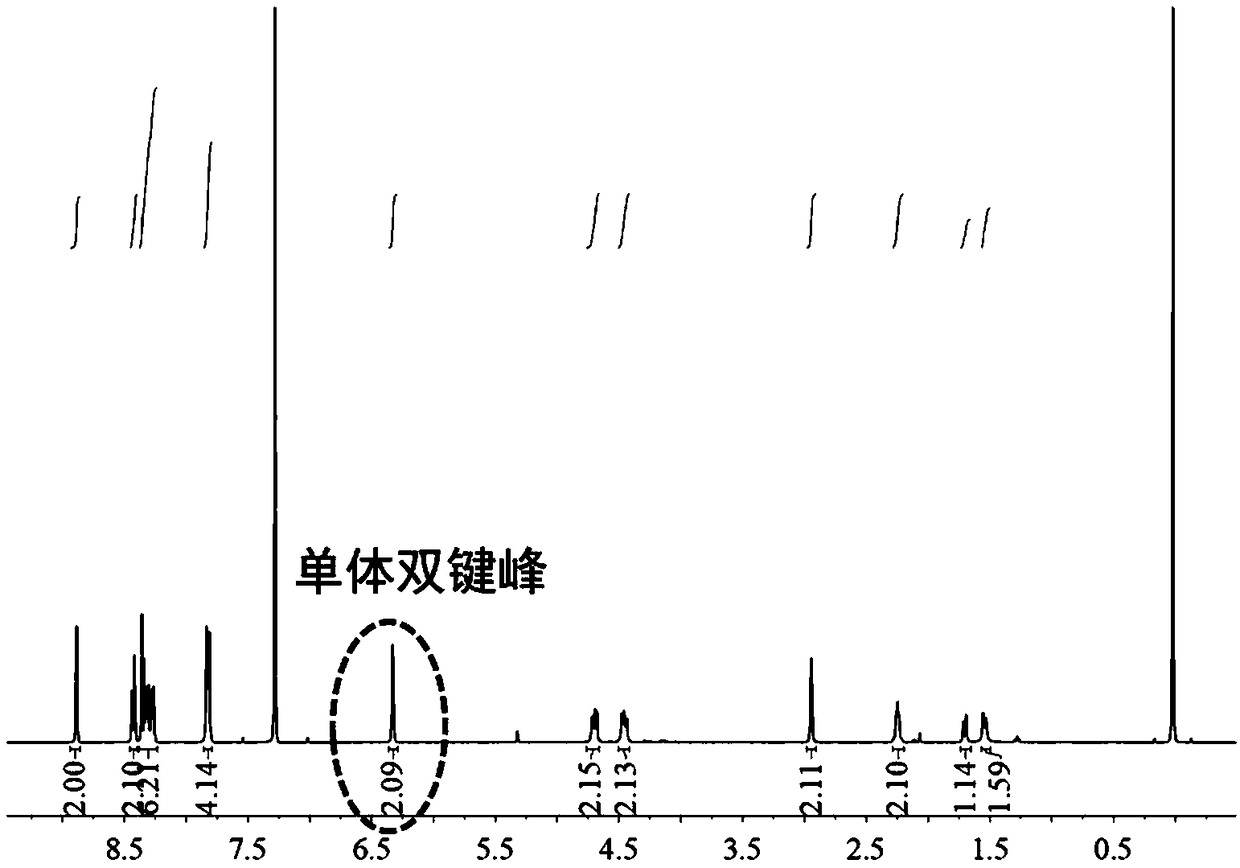
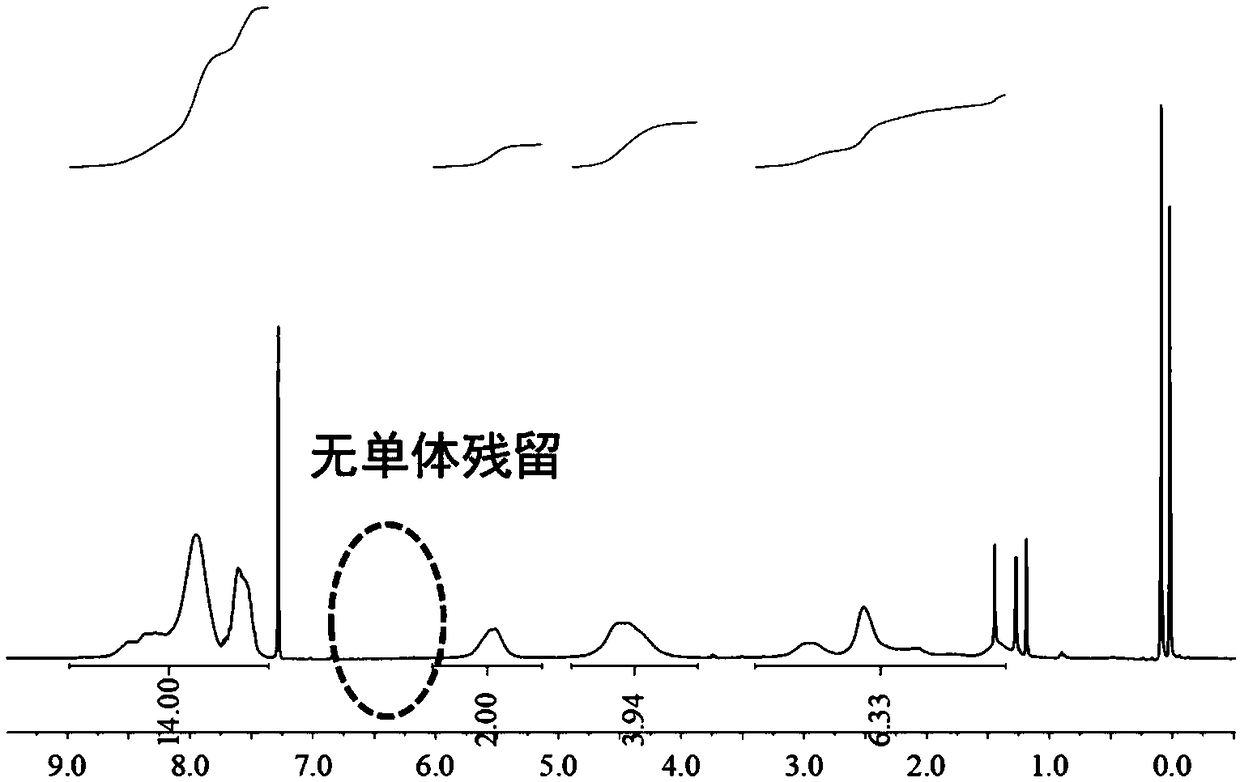
[0040] Embodiment 1-monomer synthesis
[0041]Take a 500mL check bottle with a built-in magnet; after drying, add 2.24g (14.5mmol, 1.0eqv) 5-norbornene-2,3-dimethanol, 8g 2-carboxyanthraquinone under nitrogen atmosphere (32.0mmol, 2.2eqv), 0.264g (0.220mmol, 0.15eqv) 4-dimethylaminopyridine and 6.52g (36.3mmol, 2.5eqv) 1-(3-dimethylaminopropyl)-3-ethyl carbon Diimine hydrochloride, add 400mL of dichloromethane with a syringe, and react at 20°C for 24h under magnetic stirring. The organic phase was successively washed twice with 1N dilute hydrochloric acid, saturated sodium bicarbonate, water, and saturated saline, and 300 mL of the aqueous phase was used for each extraction. Finally, the organic phase was dried with anhydrous magnesium sulfate for about half an hour, filtered through a funnel with a sand core, concentrated by rotary evaporation, and 100 mL of neutral alumina was added to fry the sample. Neutral alumina was used to pass through the column, and the eluent was ...
Embodiment 2-
[0042] Embodiment 2-monomer synthesis
[0043] Take a 500mL branch bottle with a built-in magnet. After drying, under argon atmosphere, add 2.24g (14.5mmol, 1.0eqv) 5-norbornene-2,3-dimethanol, 9.1g 2-carboxyanthraquinone (36.4mmol, 2.5eqv), 0.352g (0.29mmol, 0.15eqv) 4-dimethylaminopyridine and 7.80g (43.5mmol, 2.5eqv) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, added by syringe 400mL of dichloromethane was reacted at 30°C for 10h under magnetic stirring. The organic phase was successively washed twice with 1N dilute hydrochloric acid, saturated sodium bicarbonate, water, and saturated saline, and 300 mL of the aqueous phase was used for each extraction. Finally, the organic phase was dried with anhydrous magnesium sulfate for about half an hour, filtered through a funnel with a sand core, concentrated by rotary evaporation, and 100 mL of neutral alumina was added to fry the sample. Neutral alumina was used to pass through the column, and the eluent was pe...
Embodiment 3-
[0044] Embodiment 3-monomer synthesis
[0045] Take a 500mL branch bottle with a built-in magnet. After drying, under nitrogen atmosphere, add 2.24g (14.5mmol, 1.0eqv) 5-norbornene-2,3-dimethanol, 10.9 2-carboxyanthraquinone (43.6mmol, 3.0eqv), 0.528g (0.440 mmol, 0.30eqv) 4-dimethylaminopyridine and 10.4g (58.1mmol, 4.0eqv) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, add 400mL tetrahydrofuran with a syringe , Under magnetic stirring, react overnight at 35°C for 5h. After the organic phase was spin-dried, 400 mL of dichloromethane was added, followed by washing twice with 1N dilute hydrochloric acid, saturated sodium bicarbonate, water, and saturated saline, and 300 mL of the aqueous phase was used for each extraction. Finally, the organic phase was dried with anhydrous magnesium sulfate for about half an hour, filtered through a funnel with a sand core, concentrated by rotary evaporation, and 100 mL of neutral alumina was added to fry the sample. Neutral a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com