Bone repair material as well as preparation method and application thereof
A bone repair and reaction technology, applied in the field of regenerative medicine, can solve problems such as difficulty in achieving balance, poor mechanical properties, and fast degradation, and achieve the effect of reducing allergies and improving degradation/release time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
[0043] The preparation of embodiment 1BMP-2
[0044] In order to facilitate the experiment process and quality control, the BMP-2 used in the experiment was prepared by the applicant:
[0045] According to the rH-BMP2 amino acid and gene sequence in Genbank, the rH-BMP2 gene sequence suitable for expression in Escherichia coli was constructed, and its nucleotide sequence (including partial restriction site) was SEQ ID NO.1 in the sequence table; Insert the gene fragment into the pET plasmid vector after digestion, and transform the E. coli host after ligation; screen the positive clones and induce the expression of rH-BMP2; ferment, break up the bacteria, collect the inclusion bodies, lyse the inclusion bodies and refold the protein; anion and cation exchange layer The rH-BMP2 was obtained by analysis and purification, and electrophoresis showed a single band of about 12kDa (consistent with the reported rH-BMP2), and the amino acid sequence of the product was SEQID NO.2 in the...
Embodiment 2
[0047] The preparation of embodiment 2 modified chitosan
[0048] Chitosan modified with allyltrimethylammonium chloride:
[0049] Dissolve 3g of chitosan in 250ml of 5% (volume) acetic acid solution; under the protection of argon, heat to 90°C, add 6ml of 0.08mol / L cerium nitrate, and react for 30min; add 9ml of 50% (mass) allyl trimethyl Ammonium chloride aqueous solution, reacted for 2h; cooling, ethanol precipitation, washing, suction filtration, and vacuum drying to obtain allyltrimethylammonium chloride modified chitosan.
[0050] Product infrared spectrum detection shows 1415cm -1 (-NH), 1555cm -1 The characteristic absorption peak of (C=O) shows that allyltrimethylammonium chloride has been grafted on chitosan.
[0051] Chitosan modified with acrylamide:
[0052] Dissolve 3g chitosan solution in 250ml 5% (volume) acetic acid solution; under the protection of argon, heat to 50°C, add 5ml0.06mol / L cerium nitrate, react for 30min; add 8g acrylamide, react for 2h; cool...
Embodiment 3
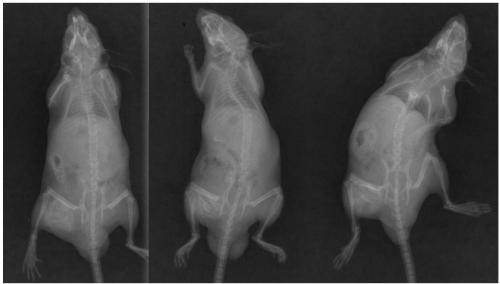
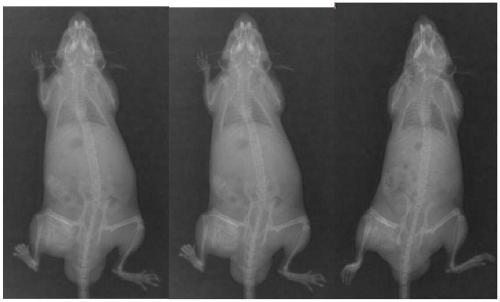
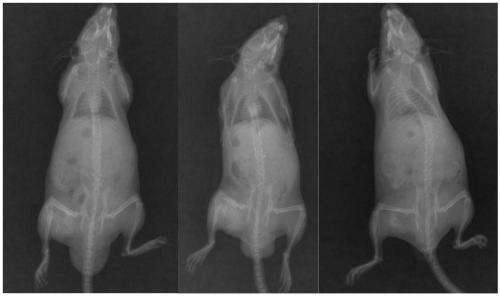
[0054] Preparation of embodiment 3 bone repair material and its basic properties
[0055] Gelatin, allyltrimethylammonium chloride or acrylamide-modified chitosan, and rH-BMP2 polypeptide components (mass ratio 1000:1000:100) of the bone repair material were stored in cold storage at 4°C when not in use.
[0056] The gelatin is dissolved in ultrapure water, the modified chitosan is added into the gelatin solution, and ground evenly; rH-BMP2 is added, mixed evenly; filled in a mold, sterilized by irradiation after vacuum freeze-drying, and the bone repair material is obtained.
[0057] The CCK-8 cell proliferation toxicity test conducted according to the kit instructions shows that the cytotoxicity of the material is CTSO-I grade, which preliminarily confirms its safety.
[0058] A control bone repair material was also prepared, in which chitosan was used instead of modified chitosan, and other processes and components remained unchanged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com