Amidohydroxycarboxylic acid/hydroximic acid compound and application thereof to ore flotation
A technology of amido hydroxycarboxylic acid and hydroxamic acid, which is applied in flotation, solid separation, etc., can solve the problems of insufficient hydrophobicity of amino acids, achieve good biocompatibility and water solubility, high flotation separation, and improve foam flotation The effect of selection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
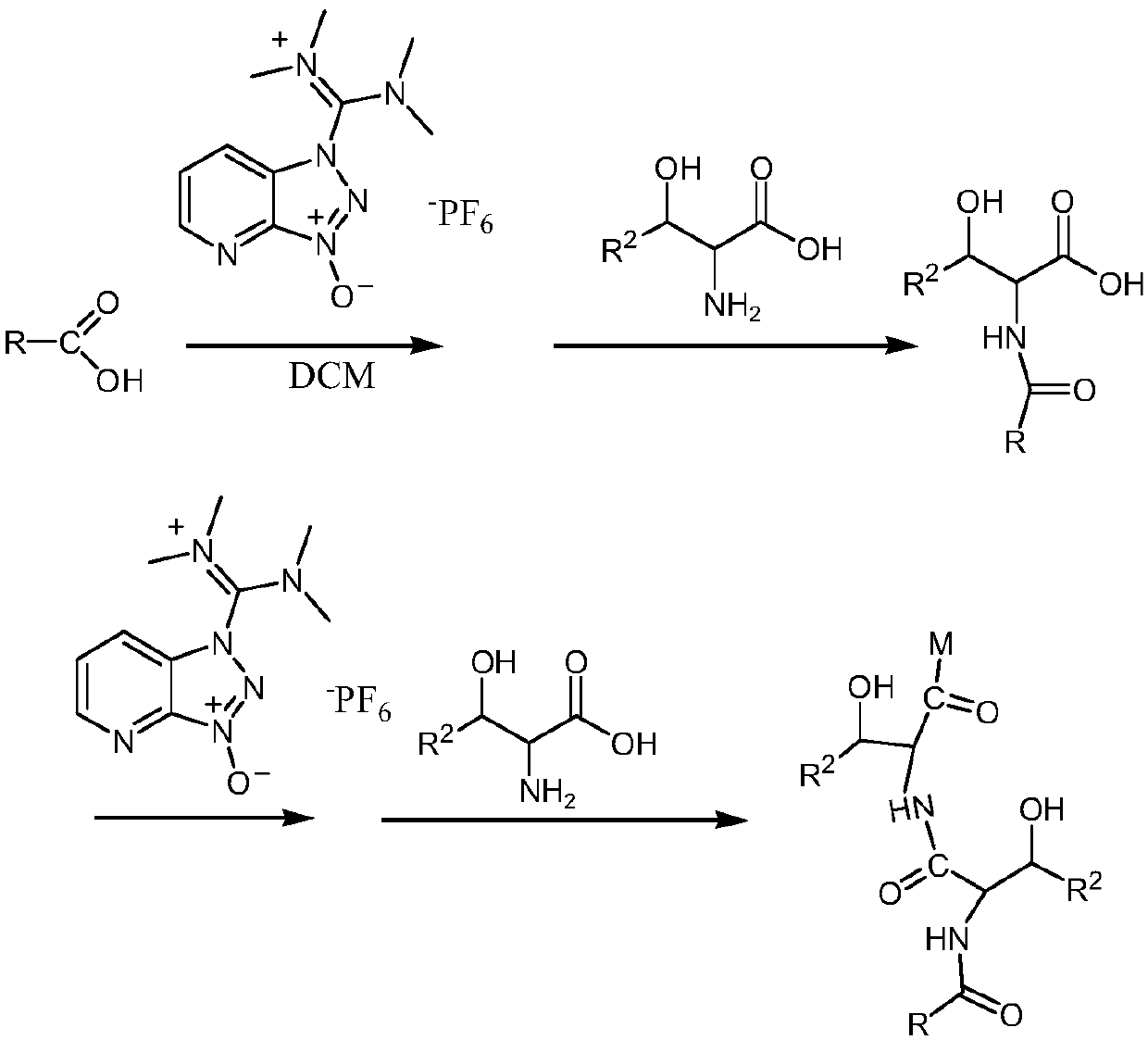
[0046] Embodiment 1: the synthetic route of amido hydroxycarboxylic acid / hydroxamic acid compound (taking 2-(2-hexadecylamino-3-hydroxybutylamino)-3-hydroxybutyric acid as an example), control reactor The temperature is 30°C, 25.64 parts of palmitic acid with a purity of 99% and 250 parts of dichloromethane are added to 38.40 parts of 2-(7-benzotriazole oxide)-N,N,N',N'-tetramethyl Add urea hexafluorophosphate to the reactor, react for 30min, then add 11.91 parts of 2-amino-3-hydroxy-butyric acid with a purity of 99% and 20.2 parts of triethylamine, and react for 10 hours; add 76.80 parts of 2-( 7-benzotriazole oxide)-N,N,N',N'-tetramethyluronium hexafluorophosphate, reacted for 30min, then added 23.82 parts of 2-amino-3-hydroxyl-butanol with a purity of 99% acid and 40.4 parts of triethylamine, react for 1h to 16h, stop the reaction, distill off dichloromethane. Wash with water, and adjust the pH of the reaction solution to 5 with 0.1M hydrochloric acid to obtain the desired...
Embodiment 2
[0047] Embodiment 2: Application of amido hydroxycarboxylic acid / hydroxamic acid compounds in tungsten ore flotation
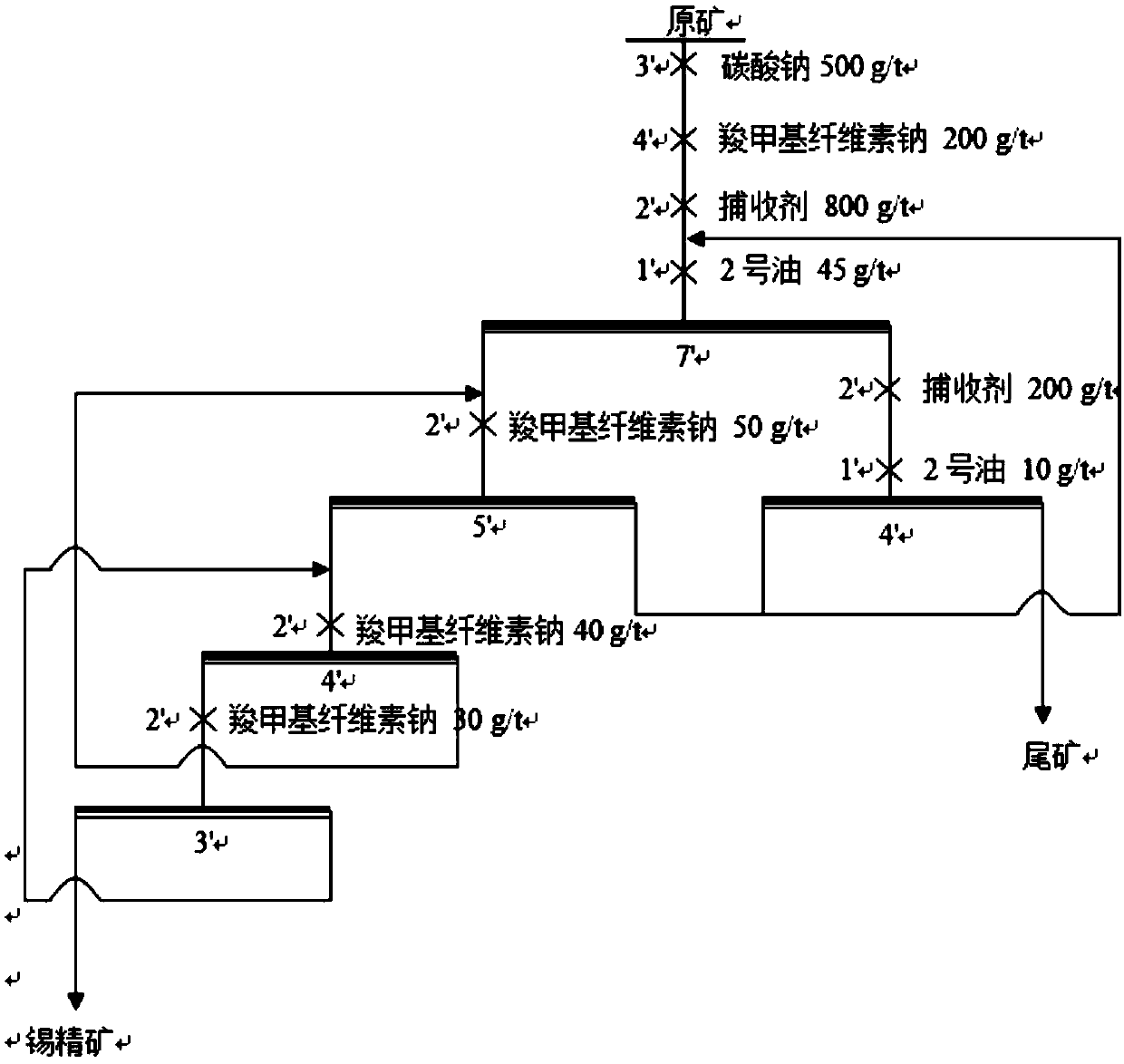
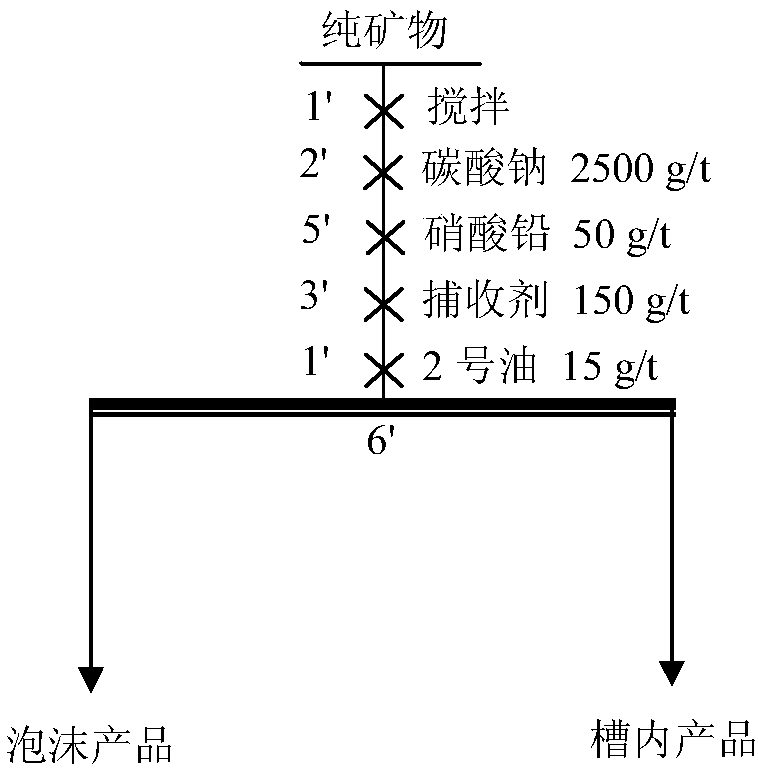
[0048] Put scheelite and wolframite pure minerals with grinding fineness -200 mesh accounting for 85% respectively in 40mL flotation tank, add 40mL water, stir for 1min to mix evenly, add 2500g / t sodium carbonate to adjust pH Value, stirred for 2mins, then added the amount of collector 100g / t, foaming agent 2 # oil 15g / t under the conditions of flotation. The flotation contrast test results of two kinds of amino acid collectors among the present invention and commonly used benzohydroxamic acid and oleic acid are shown in Table 1. Its flotation process flow chart is shown in figure 1 . It can be seen from Table 1 that the collection ability of amino acid compounds on scheelite and wolframite is significantly stronger than that of commonly used oleic acid and benzyl hydroxamic acid.
[0049] Table 1 Experimental results of flotation of pure minerals with four...
Embodiment 3
[0051] Example 3: Application of 2-(2-decaneamino-3-hydroxybutylamino)-3-hydroxybutyl hydroxamic acid in tungsten ore flotation
[0052] A sulfur beneficiation tailings containing WO in Yunnan 3 0.24%, grinding fineness -200 mesh accounts for 85%, use 3000g / t sodium carbonate to adjust pH value, 1500g / t water glass as dispersant, collector dosage 400g / t, 2# oil 40g / t Under the same conditions, after a roughing operation, tungsten crude concentrate is obtained, and the flotation of 2-(2-decaneamino-3-hydroxybutylamino)-3-hydroxybutyl hydroxamic acid and benzyl hydroxamic acid The results are shown in Table 2. It shows that 2-(2-decaneamino-3-hydroxybutylamino)-3-hydroxybutyl hydroxamic acid achieves higher recovery of tungsten than benzohydroxamic acid, and the recovery rate improves 10.52%.
[0053] Table 2 Flotation conditions and results of tungsten ore
[0054]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com