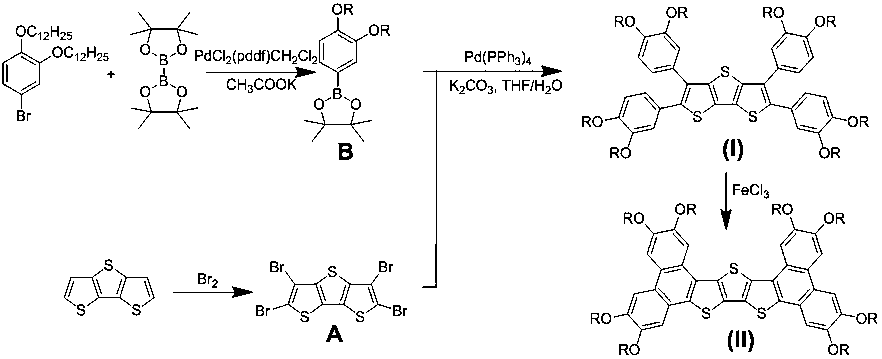
Tetraaryl-substituted bis-phenanthrene-fused compound based on dithienothiophene and preparation
A technology for bisphenanthrene fused dithiophenes and compounds, applied in the field of tetraaryl substitution and bisphenanthrene fused compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
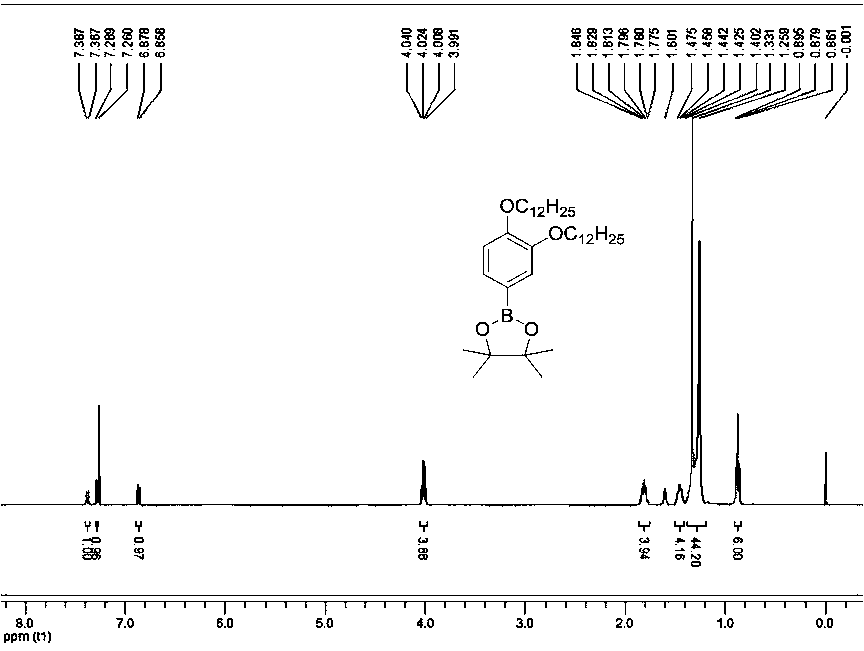
[0067] The compound provided in this example is a compound of general formula (I), wherein R is C n h 2n+1, n represents that the number of carbon atoms is 12, and its preparation process is as follows.
[0068] R is C 12 h 25 Preparation of intermediate B.
[0069] Intermediate B: .
[0070] Starting from 3,4-bis(dodecyloxy)bromobenzene, obtained in any manner, N 2 Under protection, it was mixed with biboronic acid pinacol ester and palladium catalyst ([1, 1']-bis(diphenylphosphino)ferrocenedichloropalladium dichloromethane complex) at 1:1.5:0.05 The molar ratio was added to dimethyl sulfoxide, and reacted at a temperature of 120° C. for 24 hours in the presence of potassium acetate with a molar ratio of 3:1 to the raw material. After the reaction, cool to room temperature, extract with dichloromethane, combine organic phases and dry over anhydrous magnesium sulfate, filter, and spin dry in vacuo; the resulting substance is separated and purified by silica column chro...
Embodiment 2
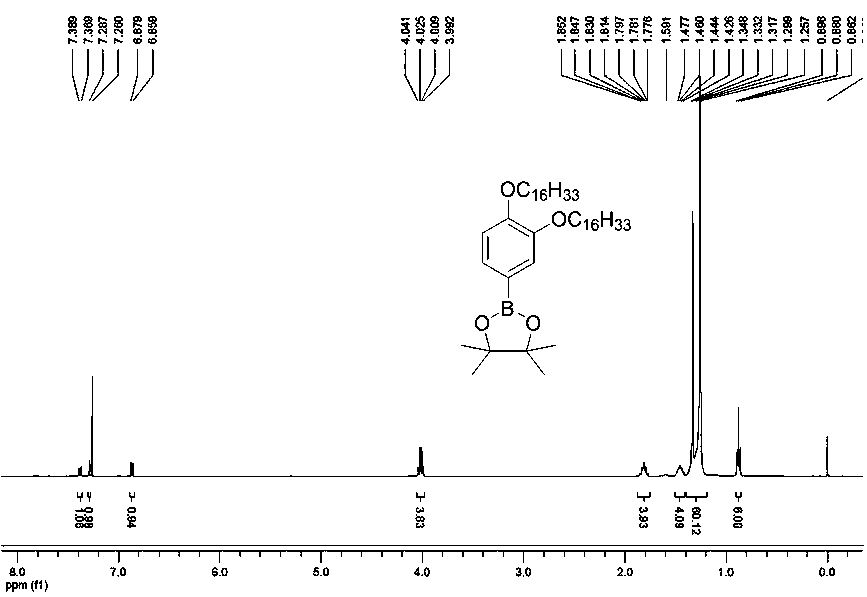
[0077] The compound provided in this example is a compound of general formula (I), wherein R is C n h 2n+1, n represents that the number of carbon atoms is 16, and its preparation process is as follows.
[0078] R is C 16 h 33 Preparation of intermediate B.
[0079] Intermediate B: .
[0080] Starting from 3,4-bis(hexadecyloxy)bromobenzene, obtained in any way, N 2 Under protection, it was mixed with biboronic acid pinacol ester and palladium catalyst ([1, 1']-bis(diphenylphosphino)ferrocenedichloropalladium dichloromethane complex) at 1:2:0.1 Add the molar ratio of 1,4-dioxane into 1,4-dioxane, and react at a temperature of 80° C. for 36 hours in the presence of potassium acetate with a molar ratio of 3:1 to the raw material. After the reaction, cool to room temperature, extract with dichloromethane, combine organic phases and dry over anhydrous magnesium sulfate, filter, and spin dry in vacuo; the resulting substance is separated and purified by silica column chromat...
Embodiment 3
[0087] The compound provided in this example is a compound of general formula (II), wherein R is C n h 2n+1, n represents that the number of carbon atoms is 12, and its preparation reaction is as follows.
[0088] General formula (Ⅱ): .
[0089] Convert R to C 12 h 25 The compound of general formula (I) was dissolved in dichloromethane, added with R as C 12 h 25 The molar ratio of the compound of general formula (I) to FeCl is 6:1 3 , room temperature reaction 3 ~ 5h. The reaction was quenched with methanol, the mixed solution was washed 3 times with distilled water, and the organic phase was spin-dried in vacuum, recrystallized 3-5 times with toluene to obtain R is C 12 h 25 The octalkoxy-substituted bisphenanthrene fused dithienothiophene compound in the general formula (II), the yield: 79%.
[0090] H NMR spectrum: 1 H NMR (CDCl3, TMS, 400 MHz). δ: 7.55 (s, 4H, ArH), 7.44 (s,2H, ArH), 7.00 (s, 2H, ArH), 4.31 (s, 4H, OCH 2 ), 4.14 (s, 12H, OCH 2 ), 1.95-2.05 (m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com