Application of aluminum-substituted ferrihydrite in heavy metal adsorption
A technology for ferrihydrite and heavy metals, applied in the direction of adsorption of water/sewage treatment, water pollutants, water/sewage treatment, etc., can solve the problem of low cadmium adsorption, achieve good removal effect, stable dosage, collaborative adsorption or fixation good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
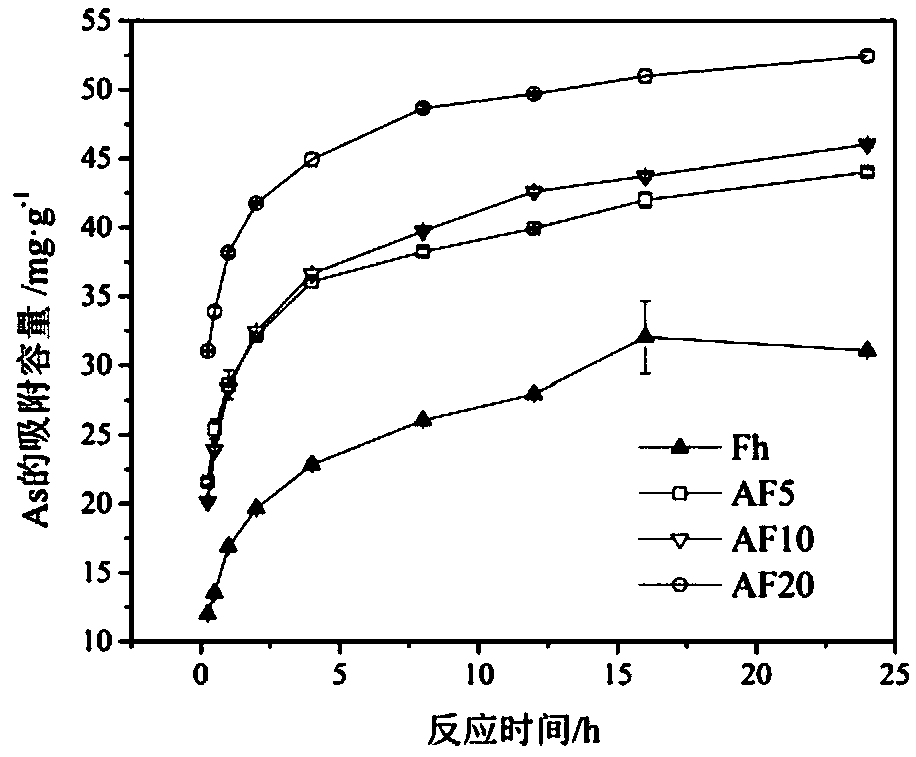
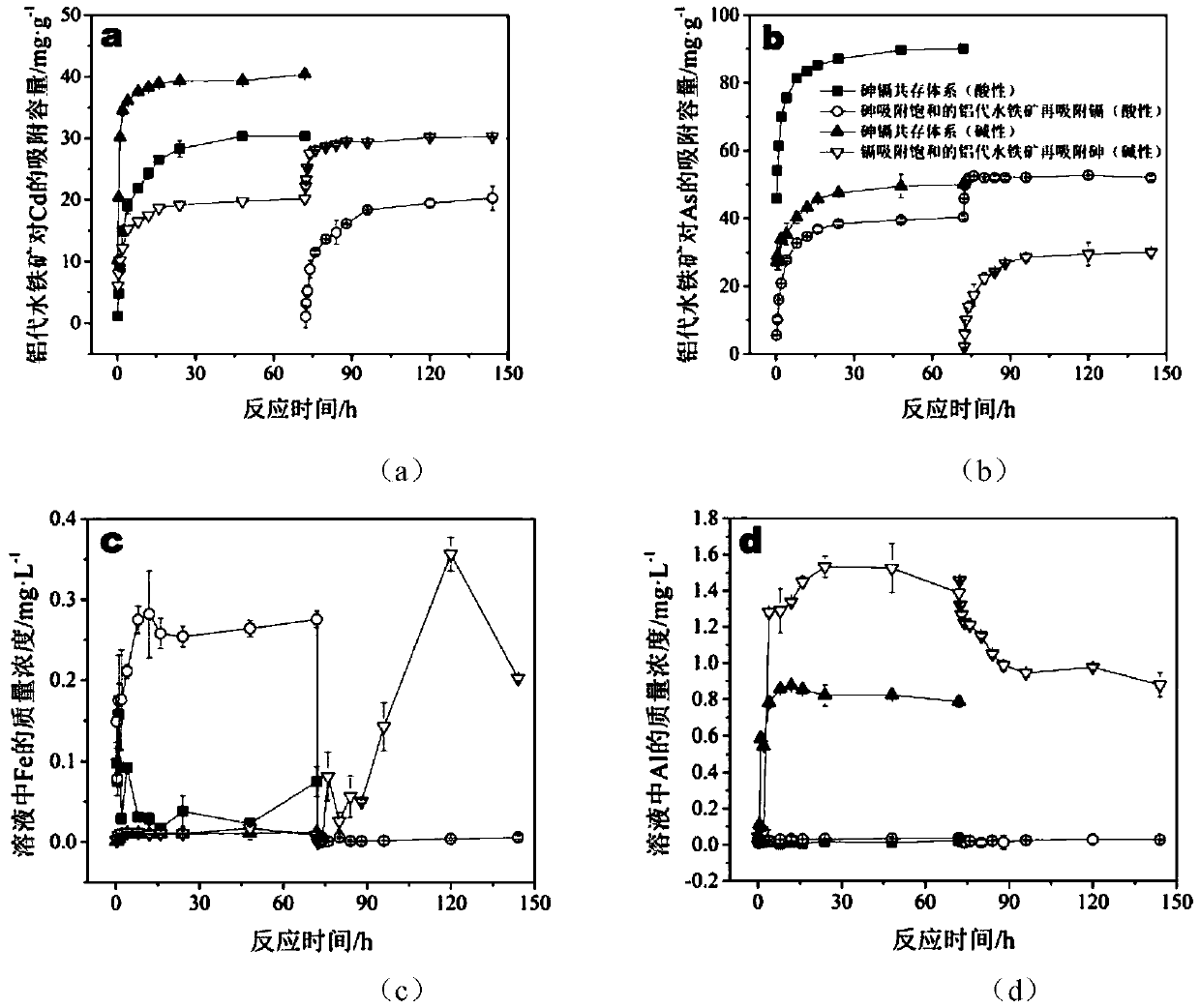
[0055] Nano-aluminum ferrihydrite was synthesized by ferric chloride and aluminum nitrate.
[0056] Configure 1mol L -1 Ferric chloride hexahydrate (A) and 1mol L -1 Aluminum nitrate nonahydrate solution (B), according to the ratio of the amount of Al / Fe+Al substance is 1:20 (take 250mL A and 13.2mL B), 1:10 (take 250mL A and 27.8mL B) and 1:5 (take 250mL A and 62.5mL B) mix the above solution, place it in a 450W power ultrasonic instrument at room temperature and stir the mixed solution at 700 rpm, and dissolve 6mol L -1 Potassium hydroxide rapid titration, the titration speed is controlled at 10mL min -1 , until the pH of the solution is 7.5, continue to maintain the above-mentioned simultaneous ultrasonic and stirring state for about 10 minutes, then stop the ultrasonic and stirring, balance the suspension for 12 hours, and place it in a 450W ultrasonic instrument at room temperature at 700 rpm Stir the suspension, adjust the pH value to 7.5, continue to maintain the abo...
Embodiment 2
[0060] Synthesize ferrihydrite by conventional method
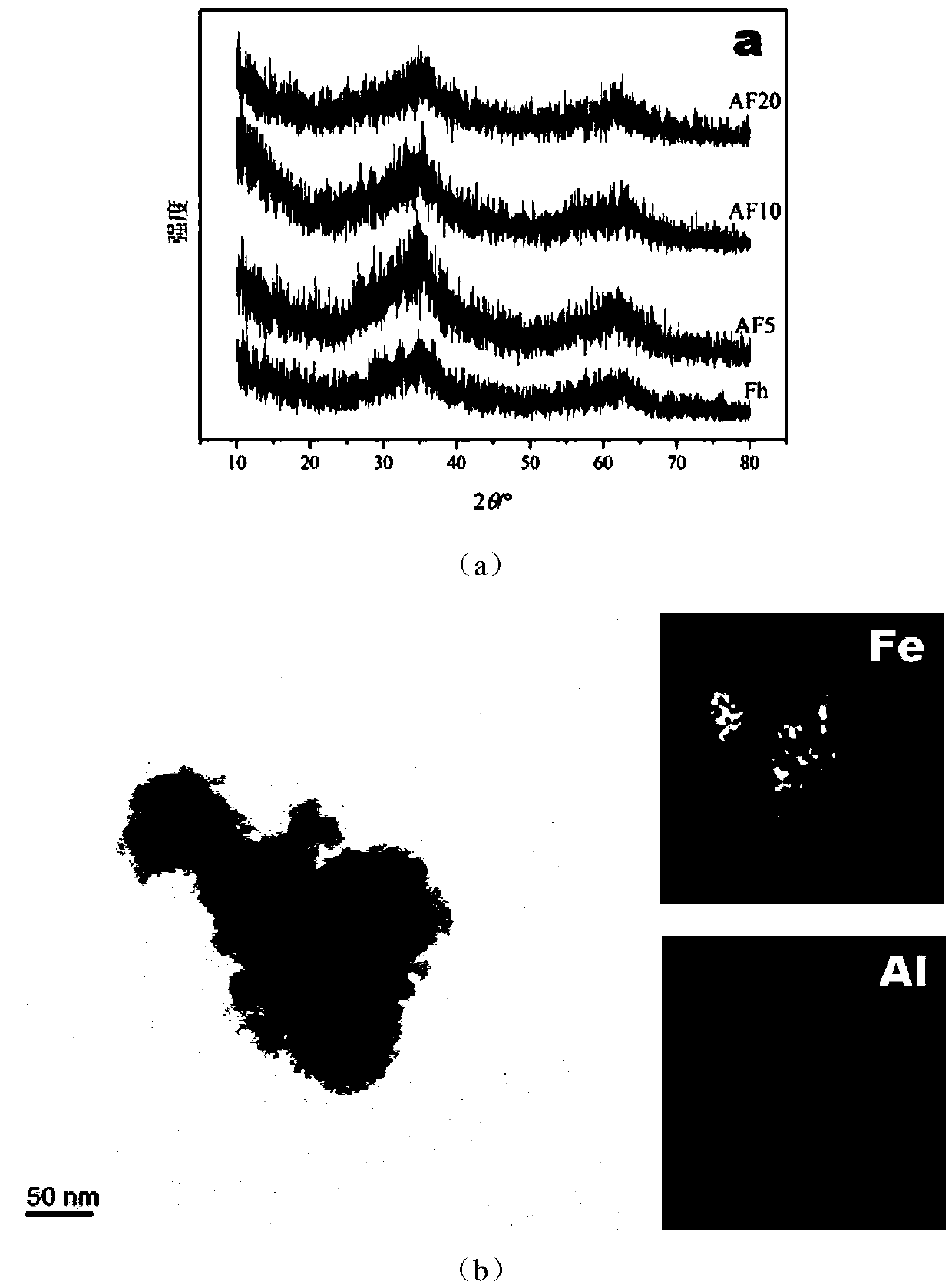
[0061] Configure 1mol L -1 Ferric chloride hexahydrate solution, take 250mL of the solution, and mix 6mol L on a stirrer at 300 rpm -1 Potassium hydroxide rapid titration, the titration speed is controlled at 5mL min -1 , until the pH of the solution is 7.5, balance the suspension for 12 hours, stir again on a stirrer at 300 rpm and adjust the pH to 7.5, then centrifuge, dialyze, freeze-dry, grind finely, and store at 4 ° C, that is Get iron ore ( figure 1 a); The particle size of ferrihydrite is larger (Table 1).
Embodiment 3
[0063] Nano-aluminum ferrihydrite was synthesized by polyferric chloride and polyaluminum sulfate.
[0064] Configuration 100g·L -1 Polyferric chloride (C) and 100g L -1 Polyaluminum sulfate (D) solution, according to the ratio of Al / Fe+Al mass fraction is 1:20 (take 250mL C and 13.2mL D), 1:10 (take 250mL C and 27.8mL D) and 1:5 (take 250mL C and 62.5mL D) mixed the above solution, placed in a 450W power ultrasonic instrument at room temperature and stirred the mixed solution at 700 rpm, and 6mol L -1 Potassium hydroxide rapid titration, the titration speed is controlled at 10mL min -1, until the pH of the solution is 7.5, continue to maintain the above-mentioned simultaneous ultrasonic and stirring state for about 10 minutes, then stop the ultrasonic and stirring, balance the suspension for 12 hours, and place it in a 450W ultrasonic instrument at room temperature at 700 rpm Stir the suspension, adjust the pH value to 7.5, continue to maintain the above-mentioned simultan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com