Two-photon fluorescence probe, and preparation method and application thereof
A two-photon fluorescence and probe technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of unfavorable modification, increase and expand its application, single use of two-photon fluorescent probes, etc., to achieve the improvement of molecular Planarity, expanding the scope of application, enhancing the effect of two-photon fluorescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
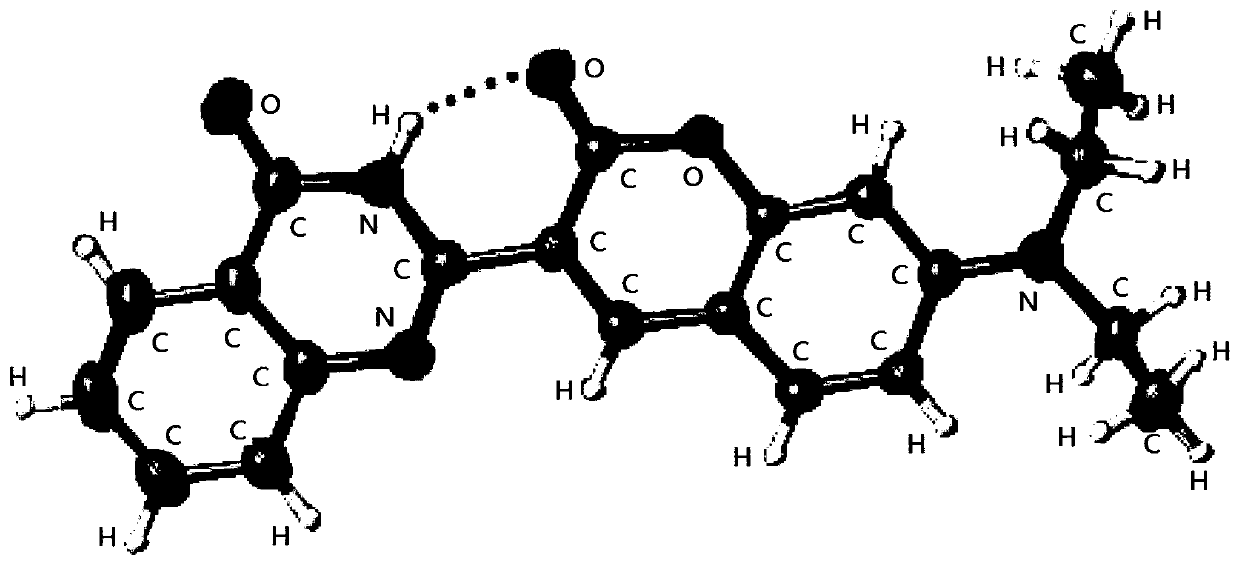
[0050] Example 1 Preparation, characterization and performance testing of the two-photon fluorescent probe 7-(diethylamino)-3-(4-hydroxyquinazolin-2-yl)coumarin.
[0051] (1) According to the reaction formula To prepare, the specific steps are: under air conditions, in a 100mL round bottom flask, add 10mmol of anthranilamide, 10mmol of 7-(diethylamino)coumarin-3-carbaldehyde, and then add wet dimethyl Sulfoxide (DMSO) 50mL, the reaction temperature is 100°C, after 10h, the materials in the reaction solution are poured into water, static phase separation, suction filtration, the obtained solid is further recrystallized with a mixed solvent of ethanol and water to obtain Two-photon fluorescent probe 7-(diethylamino)-3-(4-hydroxyquinazolin-2-yl)coumarin with a yield of 80%.
[0052] (2) According to the reaction formula To prepare, the specific steps are: under air conditions, in a 100mL round bottom flask, add 10mmol of anthranilamide, 10mmol of 7-(diethylamino)coumarin-3-ca...
Embodiment 2
[0059] The preparation, characterization and Performance Testing.
[0060] (1) According to the reaction formula Simply modify the two-photon fluorescent probe 7-(diethylamino)-3-(4-hydroxyquinazolin-2-yl)coumarin to prepare 7-(diethylamino)-3- (4-((4-nitrobenzyl)oxy)quinazolin-2-yl)coumarin, the specific steps are: in a 50mL round bottom flask, add two-photon fluorescent probe 7-(diethyl Amino)-3-(4-hydroxyquinazolin-2-yl)coumarin (0.5mmo, 180.7mg), p-nitrobenzyl bromide (1.0mmol, 216mg) and CsCO 3 (0.75mmol, 244.4mg), then add the solvent N,N-dimethylformamide (DMF) 3mL, stir under ice bath for 3h, then react at room temperature for 24h, TLC monitoring, until the reaction is complete, the reaction mixture Pour into ice water, filter with suction, dry, and then separate by column chromatography to obtain a two-photon probe with a yield of 20%.
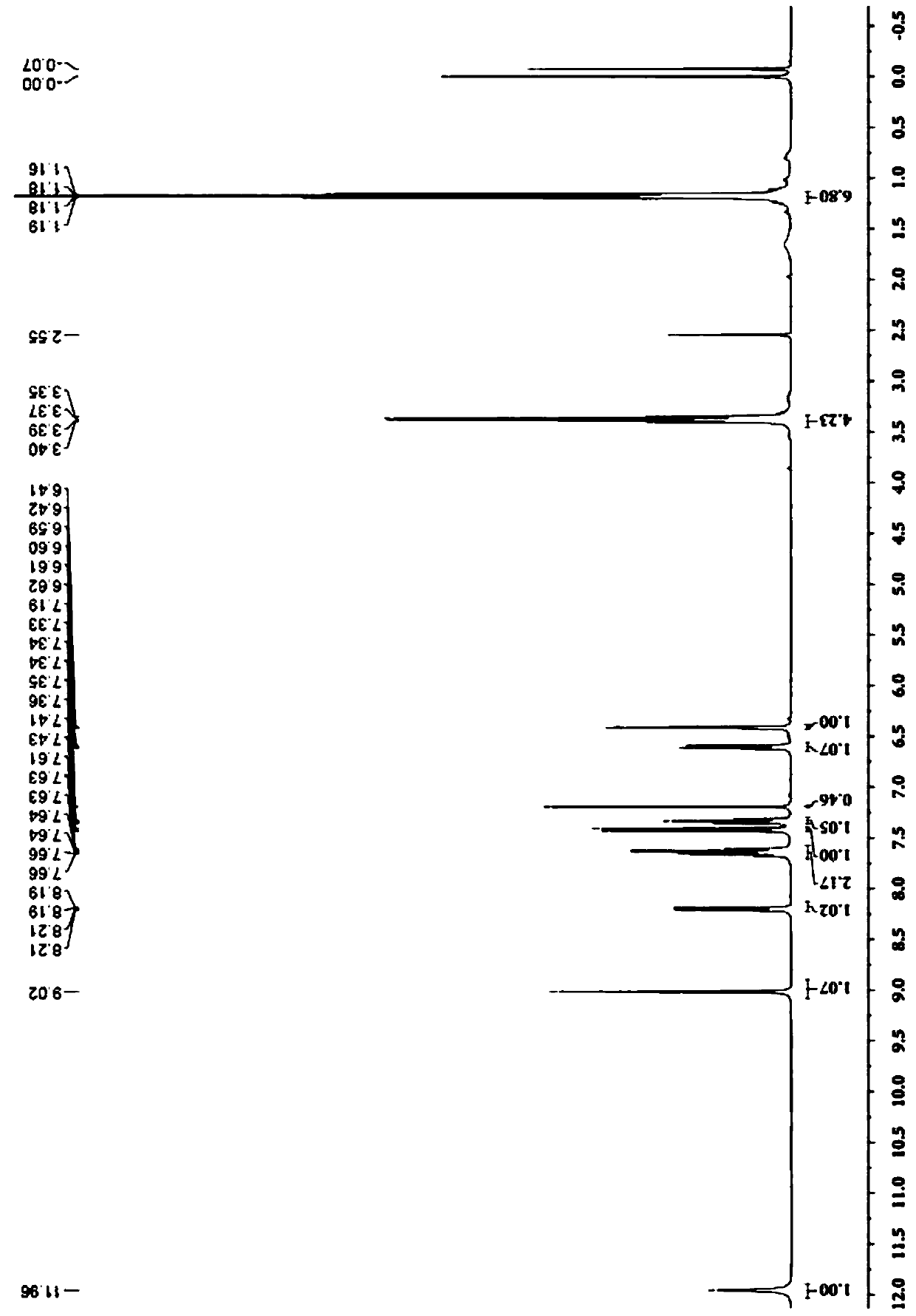
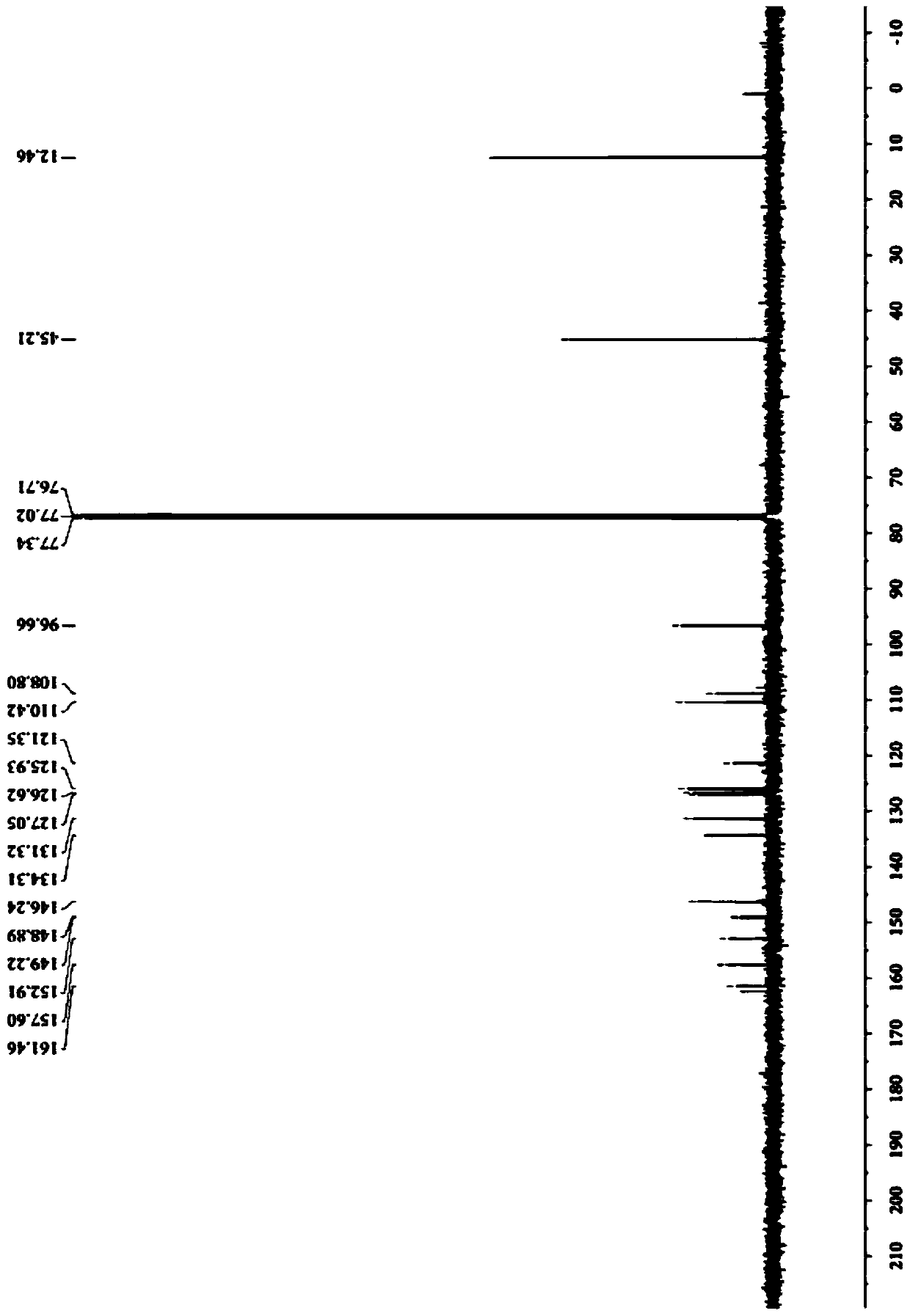
[0061] (2) carry out NMR test to the two-photon fluorescent probe that this embodiment makes, its 1 H NMR spectrum as Figure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com