Flexible liposome cosmetic containing active polypeptides and preparation method thereof
A flexible liposome, active polypeptide technology, applied in cosmetic preparations, cosmetics, cosmetic preparations, etc., can solve the problem of difficult passage of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Embodiment 1 The preparation of the flexible liposome containing active polypeptide of the present invention
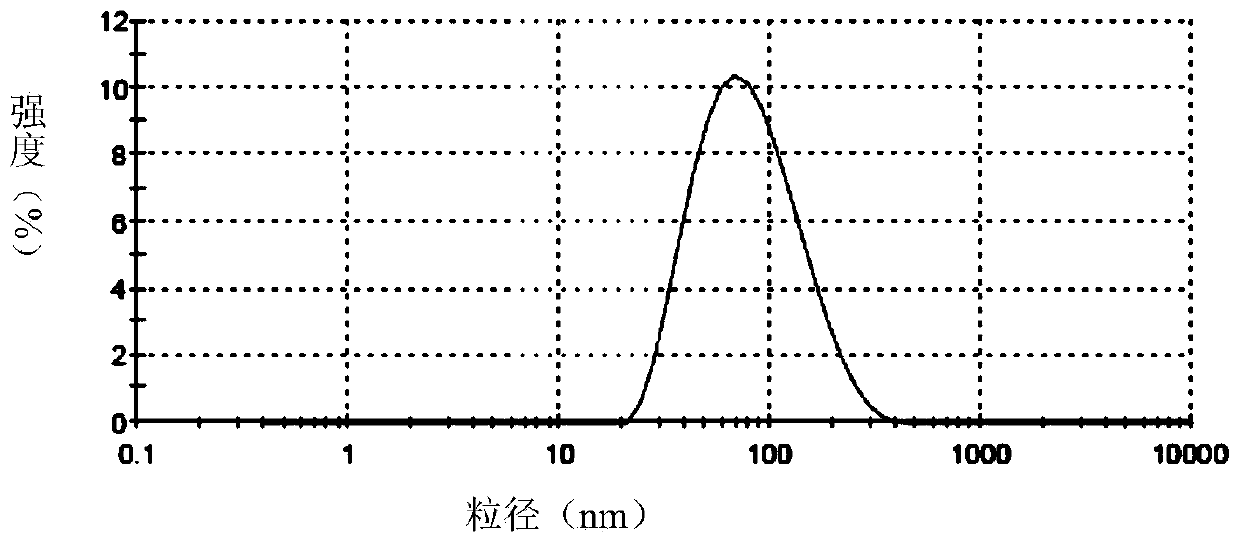
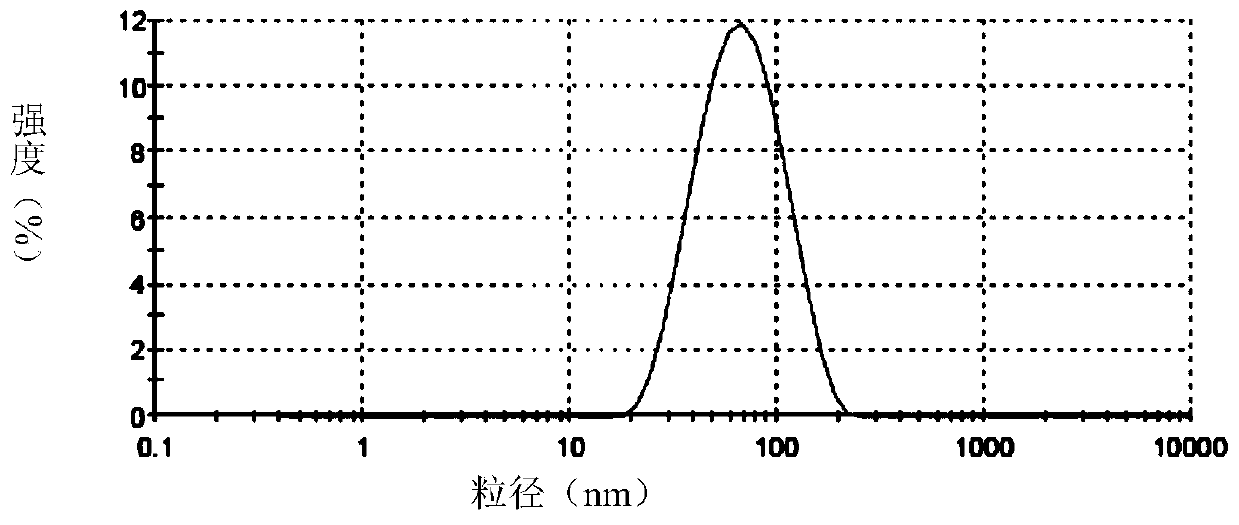
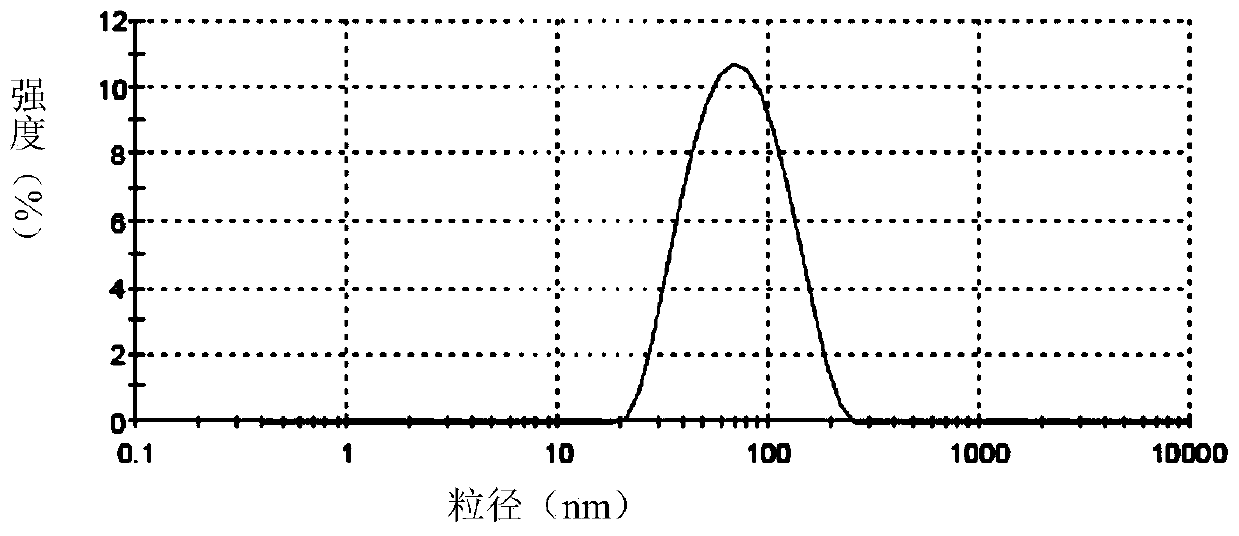
[0096] Accurately weigh soybean lecithin, sodium deoxycholate or polysorbate 80, and vitamin E according to the formula, dissolve them in chloroform / ethanol or ethanol respectively, dissolve and mix them in a 250ml pear-shaped bottle, and spin evaporate at room temperature for 2 hours, and the obtained The films were placed in a vacuum oven overnight. The next day, distilled water and appropriate amount of hydrophobized modified polypeptide were added, and a 400w probe was sonicated for 30 minutes to obtain a blank transfersome solution. Slowly add an appropriate amount of acetyl octapeptide-8 or palmitoyl tetrapeptide or palmitoyl pentapeptide or nonapeptide-1 or acetyl tetrapeptide-5 or myristoyl pentapeptide-17, etc. After incubation at room temperature for 40 minutes, the obtained The mother liquor is filtered through a 0.2 μm polycarbonate membrane for ma...
Embodiment 2
[0240] Embodiment 2 The transdermal effect verification of the flexible liposome containing active polypeptide of the present invention
[0241] The in vitro transdermal experiment adopts an improved single-chamber Franz diffusion cell, and mouse skin is used as the in vitro transdermal experimental skin to compare the transdermal absorption results of different flexible liposomes containing active polypeptides, and samples are taken at different time points. The HPLC method was used to measure the concentration of the drug in the transdermal receiving solution, and the cumulative transdermal amount was calculated.
[0242] Test example 2-1 Verification of transdermal effect of flexible liposomes containing acetyl hexapeptide-8
[0243] 1. Materials
[0244] Sample: the flexible liposome freeze-dried powder containing acetyl hexapeptide-8 (1-1,1-2,1-3) prepared by test example 1 with polysorbate 80 as surfactant, PAL-DP7 modification , 1-4) and flexible liposomes containing ...
Embodiment 3
[0321] Embodiment 3 The stability research of the flexible liposome freeze-dried powder containing active polypeptide of the present invention
[0322] The flexible liposome lyophilized powder containing active polypeptide prepared in Example 1 was subjected to stability research (including three aspects such as accelerated test and long-term test). The samples were placed in a drug stability test chamber at 37°C for accelerated testing. They were placed for 1, 2, 3, and 6 months respectively, and the properties of the freeze-dried powder were measured, including color, content, and solubility. The results are shown in Table 13. Then put the samples in a drug stability test box at 25°C for long-term tests, and place them for 3, 6, 9, 12, 15, 18, 21, and 24 months respectively to detect the color, HPLC content, and solubility of the freeze-dried powder. The results are shown in Table 14.
[0323] Table 13 Stability Accelerated Test (37° C.) of flexible liposome freeze-dried po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com