Nattokinase enteric-coated tablet and preparation method thereof
A technology of soybean kinase intestine and nattokinase, which is applied in the direction of medical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of inability to prevent thrombosis, inability to regulate blood pressure, and thrombolysis Poor effect and other problems, to avoid the damage of gastric juice, reduce the loss, and prevent the formation of thrombus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
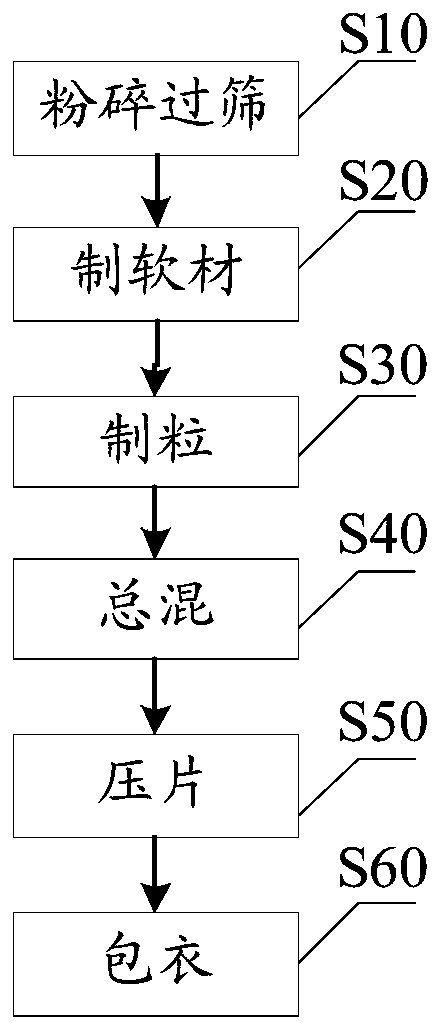
[0049] In order to prepare the above-mentioned nattokinase enteric-coated tablets, the present invention also proposes a preparation method of nattokinase enteric-coated tablets, combining figure 1 The schematic flow chart of an embodiment of the preparation method of nattokinase enteric-coated tablet provided, as can be seen, the preparation method of described nattokinase enteric-coated tablet comprises the following steps:
[0050] Step S10, crushing and sieving: take nattokinase, astragalus extract, filler, binder, lubricant, disintegrating agent and coating material, respectively crush, pass through a 100-120 mesh sieve, and set aside.
[0051] Crushing and sieving can reduce the particle size of raw and auxiliary materials, increase the specific surface area of the drug, improve the bioavailability, make the ingredients can be mixed evenly, and be easy to form into tablets. The 100-120 mesh sieve mentioned above is a standard drug sieve, and its mesh meets GB / T6003.1-2...
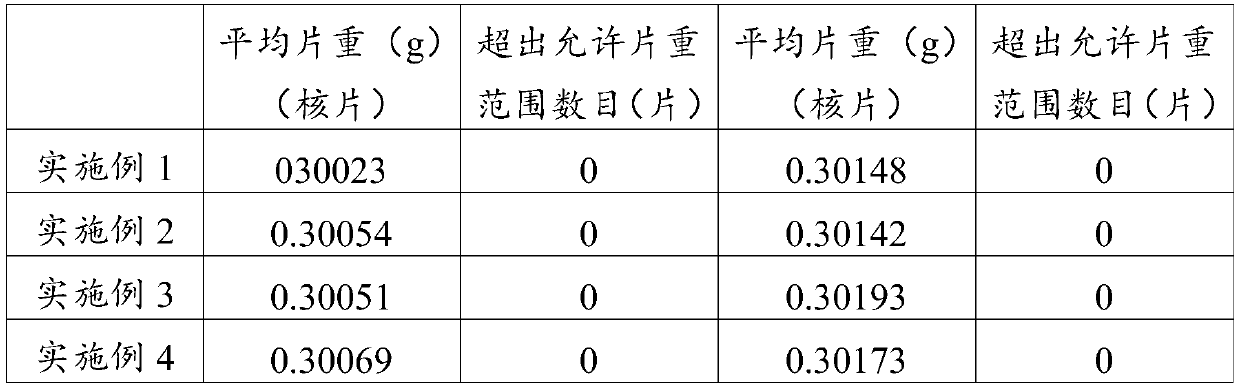
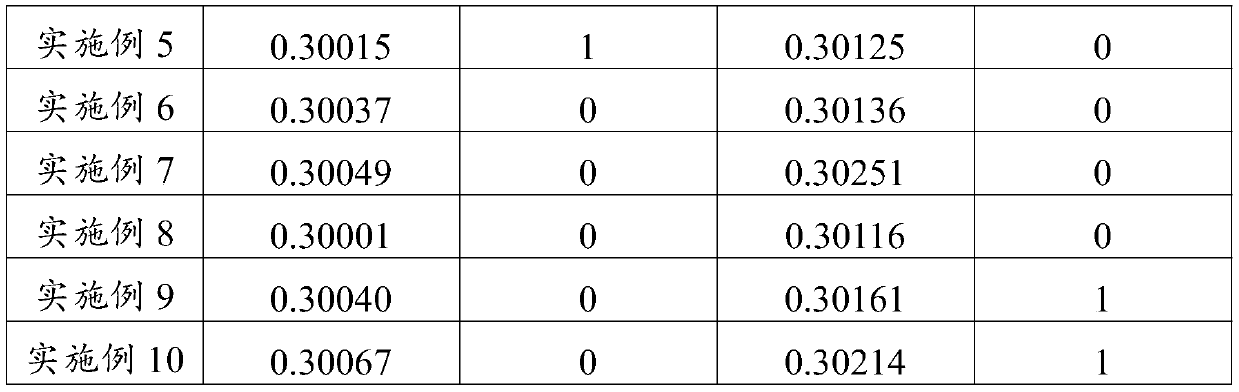
Embodiment 1
[0069] Weigh 30g of nattokinase, 10g of astragalus extract, 45g of filler (microcrystalline cellulose), 10g of binder (dextrin), and 1g of lubricant (magnesium stearate) that have been pulverized and processed through a 100-mesh sieve. , disintegrant (sodium carboxymethyl starch) 5g and coating material (acrylic resin No. II and acrylic resin No. III mixture) 4g.
[0070] Dissolve the above-mentioned nattokinase and astragalus extract in water, add part of the above-mentioned filler and mix to form a mixture with a water content of 40% (w / w), and vacuum-dry the mixture at 30°C, and then pass it for 60 Mesh sieve to obtain a mixed powder with a water content of 5% (w / w); the above-mentioned binder is dissolved in water to obtain a binder solution with a concentration of 10% (w / v); the mixed powder, binder The agent solution and the rest of the above-mentioned fillers are evenly mixed to obtain a soft material.
[0071] Sieve the soft materials to obtain wet granules, vacuum-dr...
Embodiment 2
[0076] Weigh respectively 35g of nattokinase, 15g of astragalus extract, 35g of filler (starch), 8g of binder (gelatin), 2g of lubricant (polyethylene glycol), and 2g of disintegrating agent that have been crushed and processed through a 100-mesh sieve. (Carboxymethylcellulose) 6g and coating material (cellulose acetate phthalate) 5g.
[0077] Dissolve the above-mentioned nattokinase and astragalus extract in water, add part of the above-mentioned filler and mix to form a mixture with a water content of 50% (w / w). The mixture is vacuum-dried at 25°C, and then dried for 50 Mesh sieve to obtain a mixed powder with a water content of 3% (w / w); the above binder is dissolved in water to obtain a binder solution with a concentration of 5% (w / v); the mixed powder, binder The agent solution and the rest of the above-mentioned fillers are evenly mixed to obtain a soft material.
[0078] Sieve the soft materials to obtain wet granules, dry them under vacuum microwave at 25°C, and then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com