A solid polymer electrolyte and its application in lithium metal batteries
A solid polymer, polymer technology, used in secondary batteries, secondary battery manufacturing, secondary battery repair/maintenance, etc. Weight, improved cycle performance and safety performance, effect of high ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
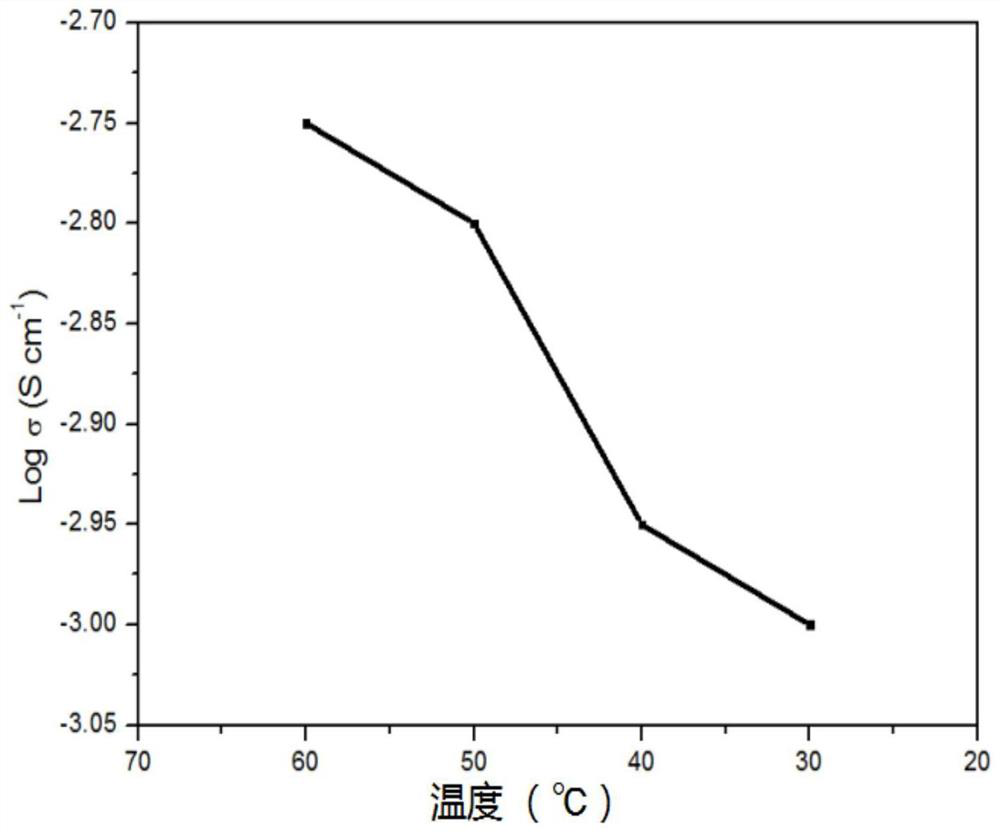
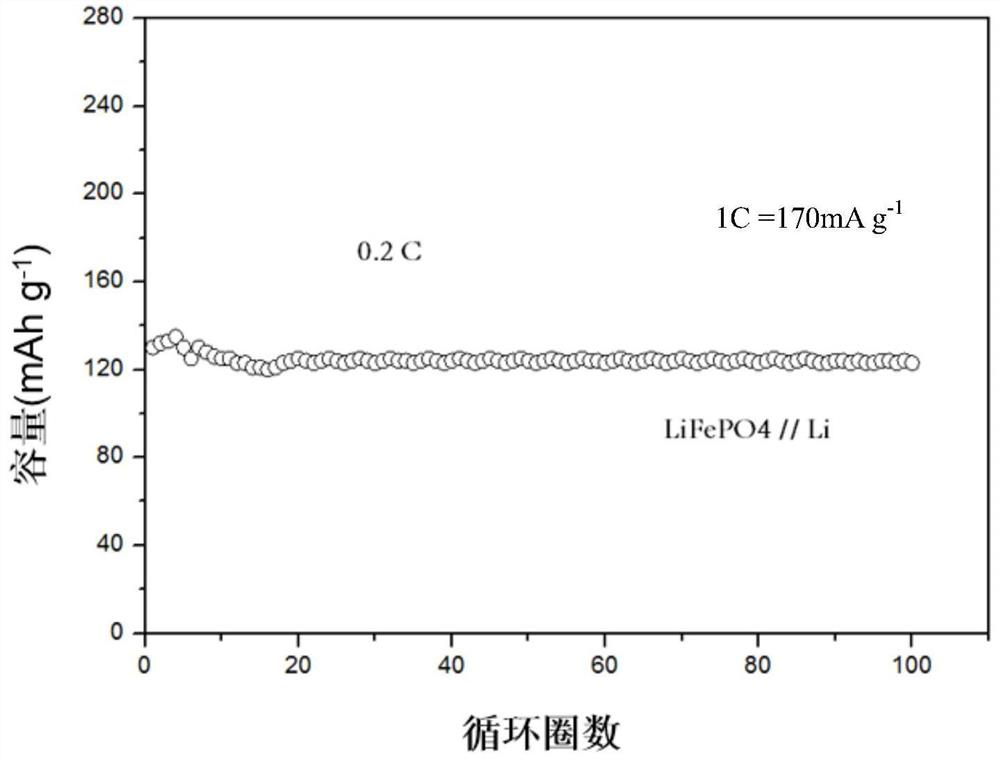
[0025] This example provides a solid polymer electrolyte and its application, as follows: Dissolve tris(4-aminophenyl)amine, tris(4-formylphenyl)amine and glacial acetic acid in dioxane, put In a sealed quartz glass tube, degas under the condition of liquid nitrogen bath, seal under Ar condition, and react at 120°C. After the reaction produces a solid, purify, disperse in DMF, add 2-chloro-1,1,3-trimethoxypropane, ionize, purify and dry, the resulting solid is dispersed in an aqueous solution, add 1mol / L bistrifluoromethyl Lithium sulfonyl imide solution, anion exchange, get anion as TFSI - COF-IL material, and then immerse it in 1mol / L lithium hexafluorophosphate solution at room temperature for 12 hours, then filter, separate the solid, dry, and then use a ball mill to grind it evenly, and press it into a tablet to obtain a sheet-shaped solid electrolyte. Assembled button cell; the ratio of the amount of substances of the three (4-aminophenyl) amine, three (4-formylphenyl) ...
example 2
[0029] This example provides a solid polymer electrolyte and its application, specifically as follows: Dissolve tris(4-aminophenyl)amine, tris(4-formylphenyl)amine, and strong protonic acid fluorosulfonic acid in mesitylene , placed in a sealed quartz glass tube, degassed under liquid nitrogen bath conditions, sealed under Ar conditions, and reacted at 120°C. After the reaction generates a solid, purify, disperse in DMF, add chloroacetaldehyde dimethyl acetal, ionize, purify and dry, and the obtained solid is dispersed in an aqueous solution, add 1 mol / L lithium bistrifluoromethanesulfonimide (LiTFSI) solution, anion exchanged to obtain anion as TFSI - COF-IL material, and then immerse it in 1mol / L lithium bistrifluoromethanesulfonimide solution at room temperature for 12 hours, then filter, separate the solid, dry, and then use a ball mill to grind evenly, and press into tablets. A sheet-shaped solid electrolyte is obtained, and the battery is assembled to obtain a button ba...
example 3
[0032] This example provides a solid polymer electrolyte and its application, specifically as follows: Dissolve tris(4-aminophenyl)amine, tris(4-formylphenyl)amine and noble metal salt in N,N-dimethylformaldehyde In the amide, put it into a sealed quartz glass tube, degas it under the condition of liquid nitrogen bath, seal it under the condition of Ar, and react at 120°C. After the reaction produces a solid, purify it, disperse it in DMF, add epoxybromobutane, ionize it, purify and dry it, and disperse the obtained solid in an aqueous solution, add a 1mol / L difluorooxalate lithium borate solution, and perform anion exchange to obtain an anion as TFSI - COF-IL material, and then immerse it in 1mol / L lithium trifluoromethanesulfonate solution at room temperature for 12 hours, then filter, separate the solid, dry, and then use a ball mill to grind evenly, and press into tablets to obtain flakes The solid electrolyte is used to assemble the battery to obtain a button battery; the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com