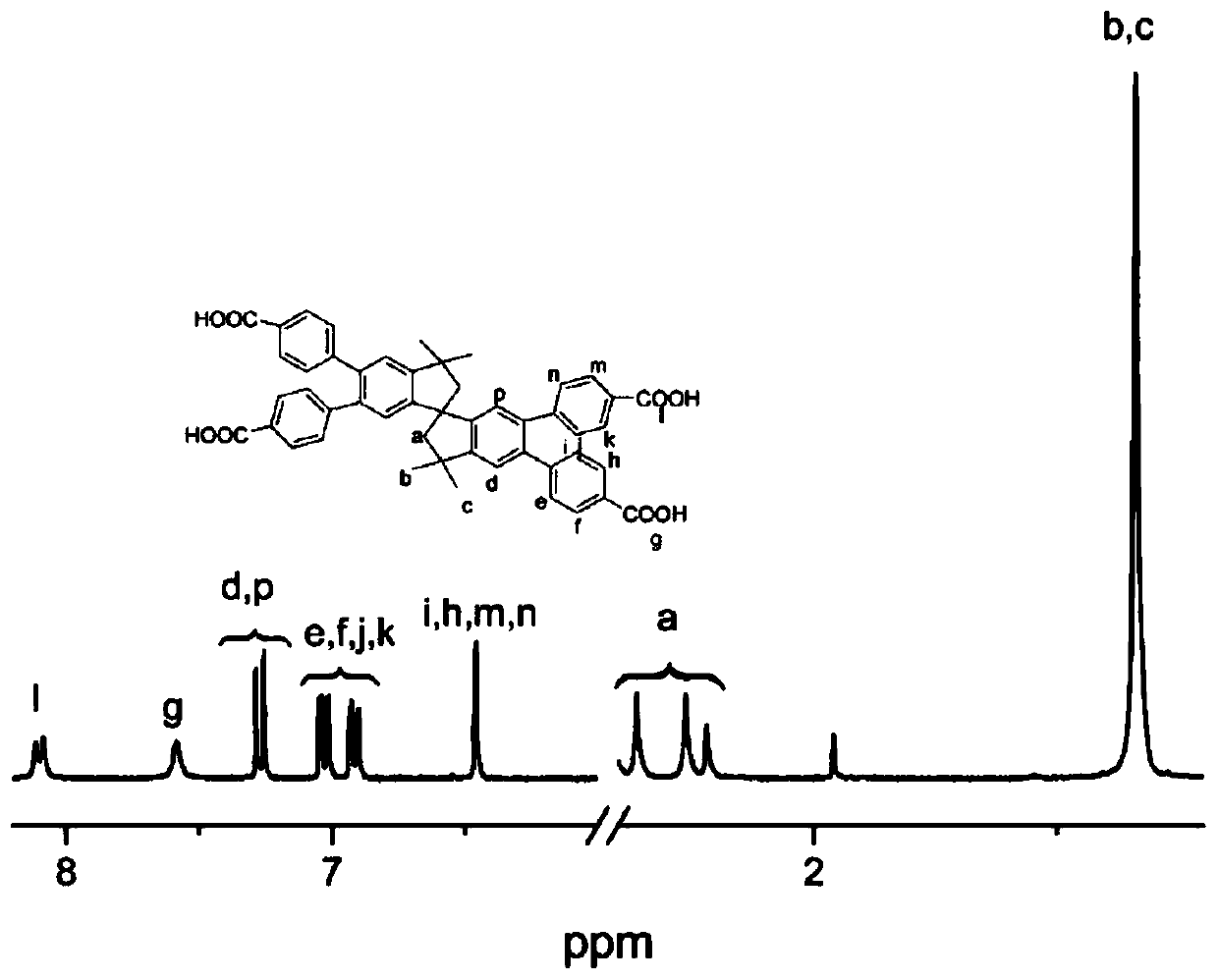
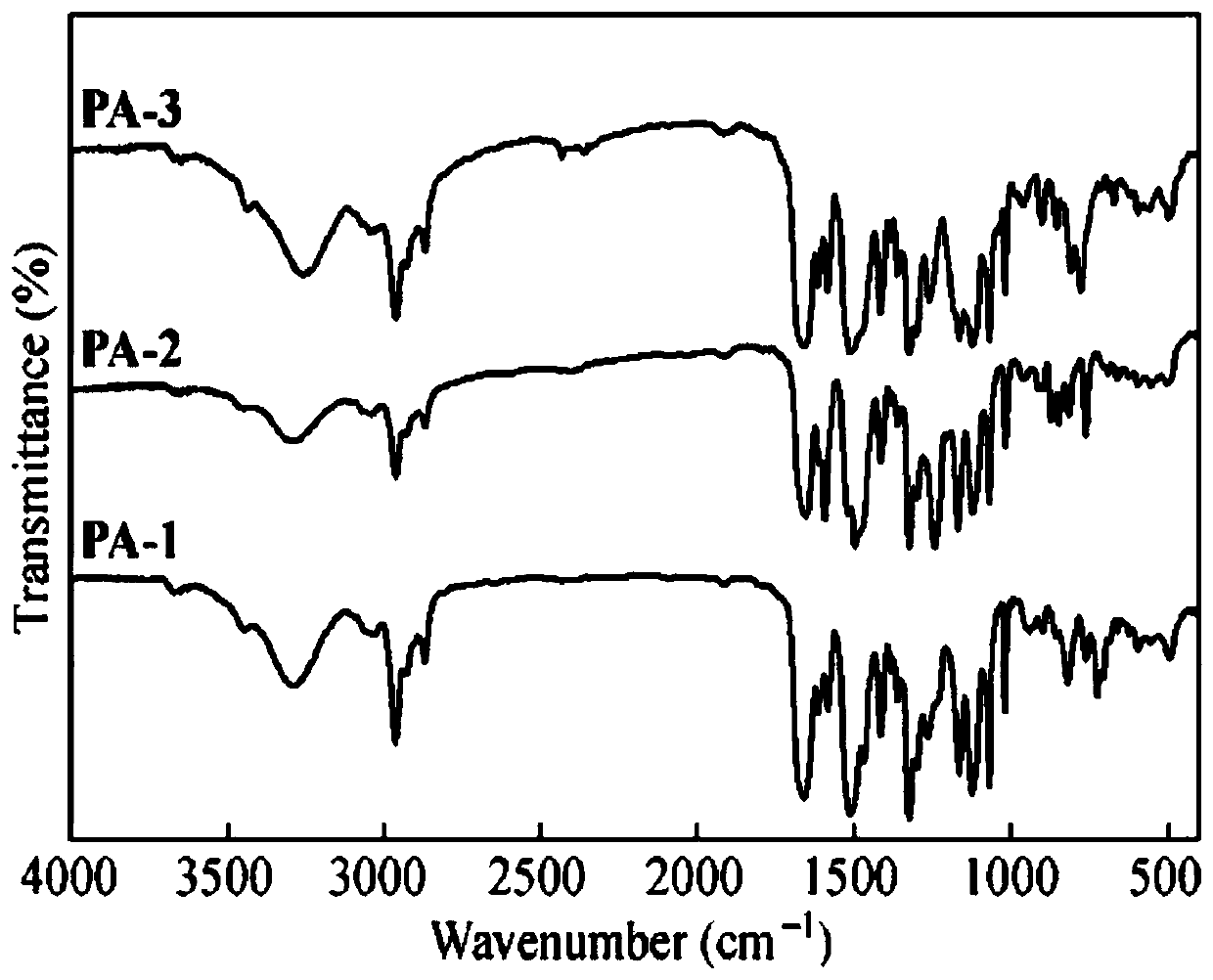
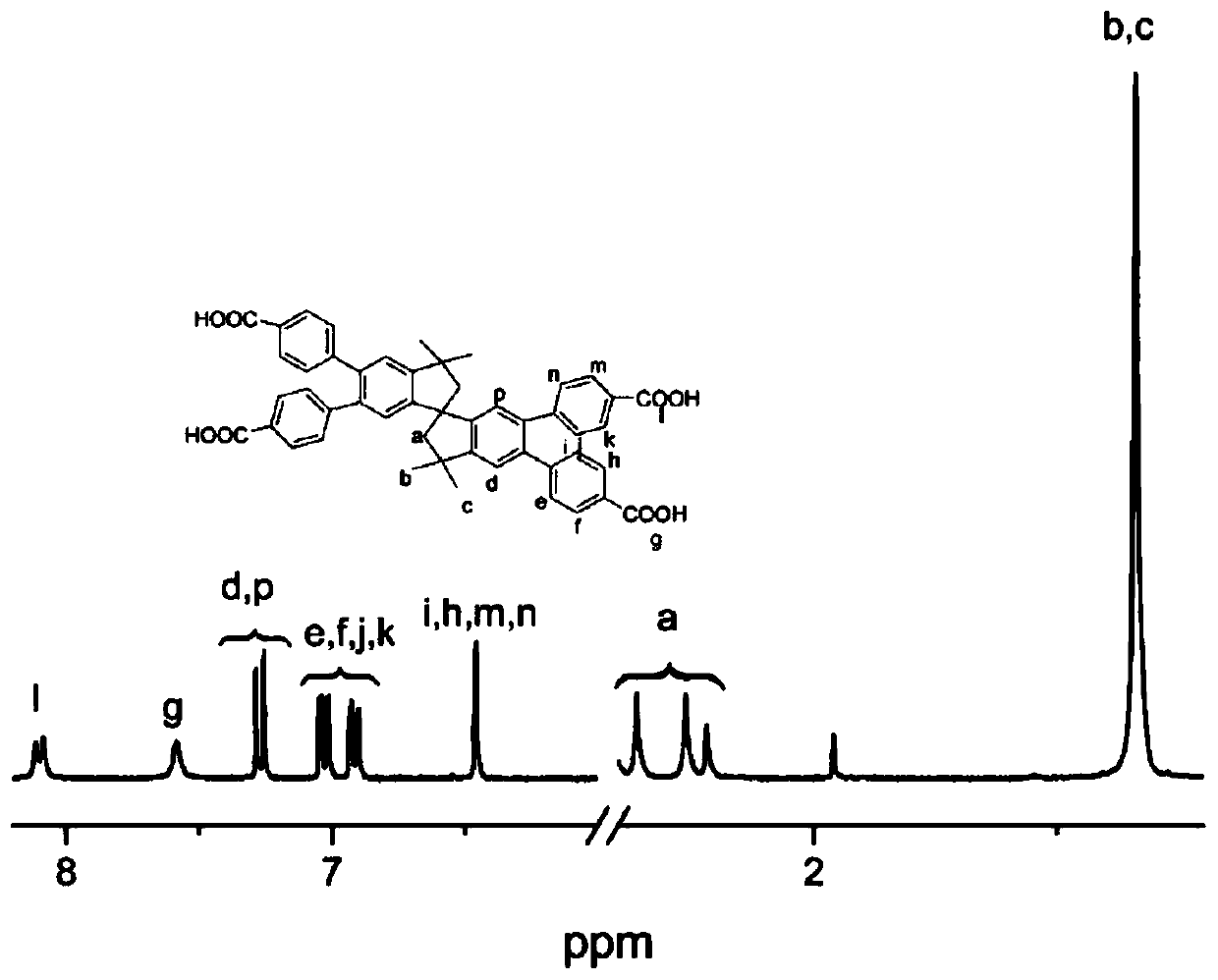
Polyacid monomer and preparation method thereof, polyamide and preparation method thereof, and polyamide film
A technology of acid monomers and polyamides, applied in the field of organic synthesis, can solve problems such as selectivity reduction, achieve good solubility, increase free volume and flexibility, and improve poor solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The present invention also provides a method for preparing the polyacid monomer described in the above technical scheme, comprising the following steps:
[0040] When the multi-acid monomer has a structure shown in formula I, the preparation method of the multi-acid monomer comprises the following steps:
[0041] (1) cooling after heating the acetone solution of hydrogen iodide and the acetic acid solution of catechol to reflux to obtain a supersaturated solution, then carrying out hydrothermal treatment to the supersaturated solution to separate out spirotetraphenol; Phenol has the structure shown in formula III:
[0042]
[0043] (2) The spiro tetraphenol obtained in the step (1) and p-fluorobenzonitrile are subjected to a substitution reaction in the presence of a catalyst and an organic solvent to obtain a tetracyano spiro compound, and the catalyst includes salt of wormwood and carbonic acid One or both of cesium; the tetracyano spiro compound has a structure s...
Embodiment 1
[0100] 85g of HI aqueous solution with a mass concentration of 99% was added to 15mL of acetone to form a mixed solution; The mass concentration of catechol is 48%, and the mass concentration of acetic acid in the catechol acetic acid aqueous solution is 40%. The obtained solution was heated to reflux at 120°C for 10 hours, then cooled to room temperature to obtain a supersaturated solution, and the obtained supersaturated solution was hydrothermally crystallized to precipitate white microcrystalline compounds at 220°C and 0.3GPa under high temperature and pressure, filtered, and used ice Acetic acid and dichloromethane were alternately washed three times to obtain 18.0432 g of spiro tetraphenolic compounds.
[0101] In a 250mL three-necked flask equipped with a mechanical stirring device, add 15mmol of the prepared spirocyclic tetraphenol compound, 64mmol of p-fluorobenzonitrile, 12mL of N-methylpyrrolidone, the total solid content of the reaction system is 15%, and stir Unt...
Embodiment 2
[0108] The HI aqueous solution that 5g mass content is 99% is joined in the acetone of 10mL to form mixed solution, the mixed solution that forms joins in the 100mL acetic acid solution that contains 105mmol pyrocatechol, the mass concentration of acetic acid in the acetic acid solution is 40%, then Then add in the 100mL acetic acid solution that contains 150mmol hydroxybenzene, the mass concentration of acetic acid in the acetic acid solution is 40%. Stirring, under the protection of sufficient nitrogen, heating and reflux for 15h, then cooling to room temperature to obtain a supersaturated solution, using the hydrothermal crystallization method to precipitate white microcrystalline compounds at 240°C and 0.5GPa high temperature and high pressure, Filtrate, and alternately wash three times with glacial acetic acid and dichloromethane to obtain a spirocyclic triphenol compound.
[0109] In a 250mL three-necked flask equipped with a mechanical stirring device, add 15mmol of spi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| osmotic coefficient | aaaaa | aaaaa |
| osmotic coefficient | aaaaa | aaaaa |
| osmotic coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap