Production method and application of micron-sized supported difunctional polymer brush with nitroxide free radicals and sulfonic groups
A technology of nitroxide free radicals and polymer brushes, which is applied in the preparation of polycarboxylate superplasticizer active macromonomers, bifunctional polymer brushes, and synthesis of polyethylene glycol monomethyl ether acrylate, which can solve the problem of nitrogen oxides Free radicals have no effect of polymerization inhibition, there is no recovery of polymerization inhibitors and acid catalysts, and poor dispersibility, etc., to achieve the effects of easy recycling, large particle size, and improved compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
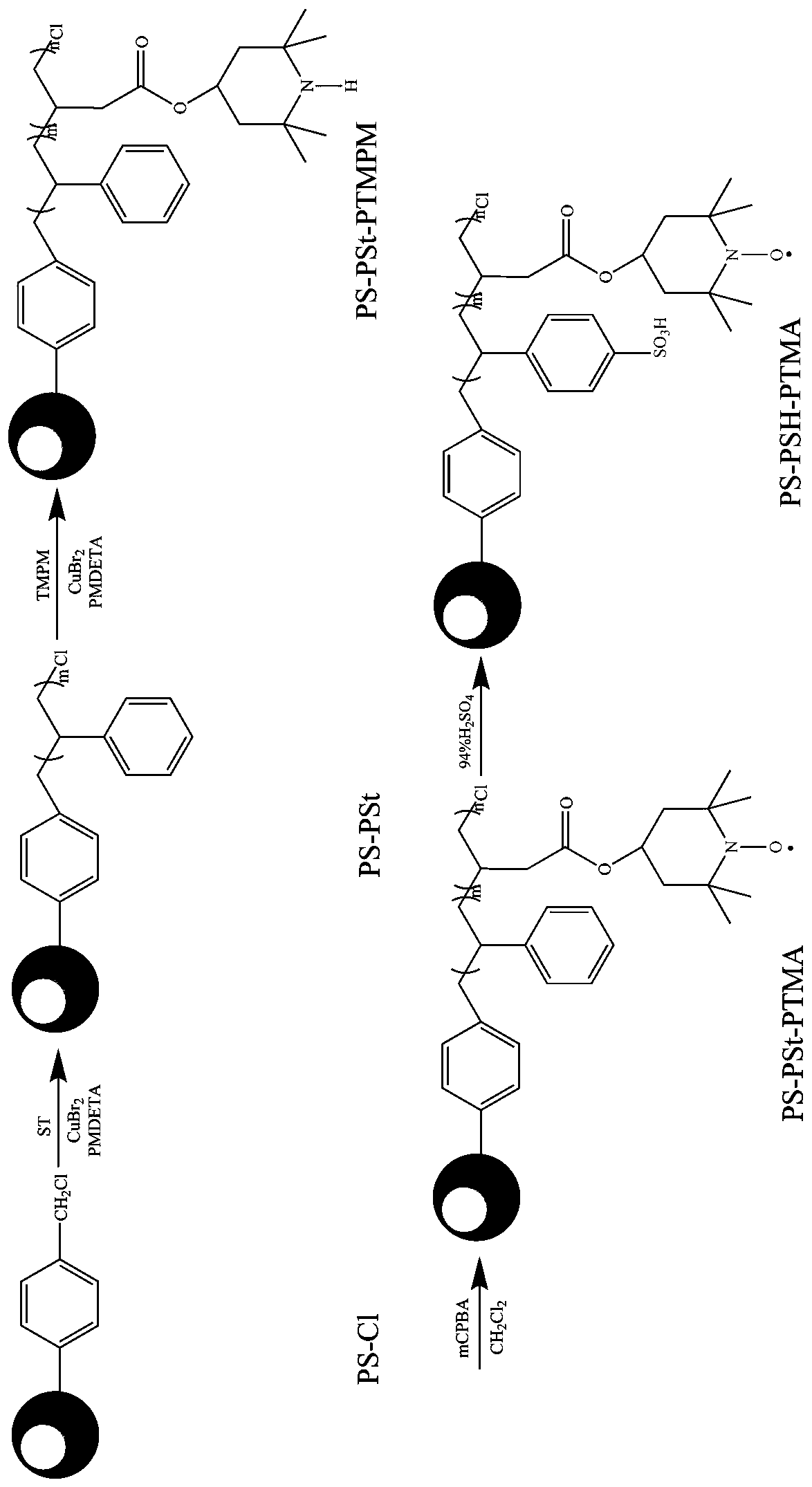
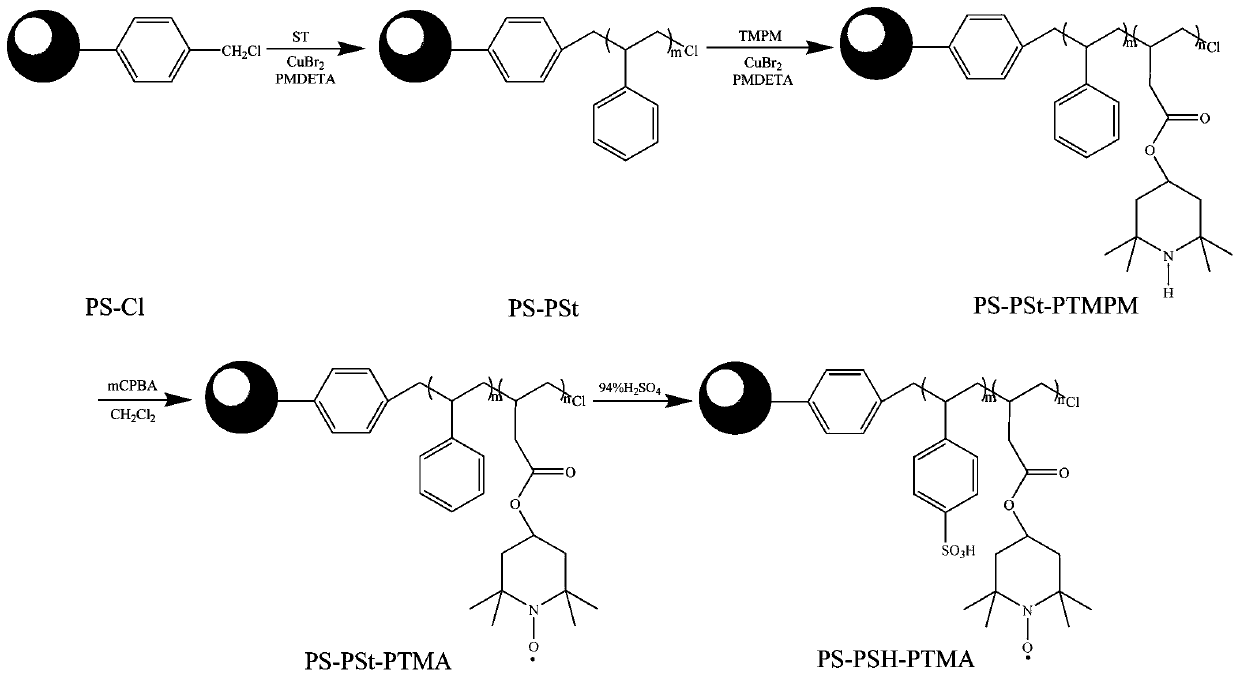
[0027] 1. Preparation of micron-sized bifunctional polymer brushes containing nitroxide radicals and sulfonic acid groups
[0028] (1) Add CuBr to a 100mL three-neck flask 2 (0.0250 g, 0.112 mmol), solvent anisole (30 mL) and magnetic rotor, ultrasonic vibration 2After complete dissolution, add a condenser tube and a thermometer to the three-necked flask, and set the temperature to 40°C. Under nitrogen atmosphere, St (11.6648 g, 112 mmol), PMDETA (0.23 mL, 1.12 mmol), PS-Cl (1 g, 1.12 mmol) and stannous octoate ( 0.36 mL, 1.12 mmol). The reaction temperature was set to 110 °C and reacted for 9 h. After the reaction was completed, the reaction solution was poured into a beaker. Let stand for 12 h, remove the supernatant by filtration, and dry in vacuum until constant weight. The resulting product is the homopolymer brush PS-PSt.
[0029] (2) Add CuBr to a 100 mL three-neck flask 2 (0.0098 g, 0.04 mmol), solvent anisole (30 mL) and magnetic rotor, ultrasonic vibration 2...
Embodiment 2
[0037] 1. Preparation of micron-sized double-tapping functional polymer brushes containing nitroxide radicals and sulfonic acid groups
[0038] (1) Add the catalyst CuBr into a 100 mL three-necked flask 2 (0.0250 g, 0.112 mmol), solvent anisole (30mL) and magnetic rotor, ultrasonic vibration 2 After complete dissolution, add a condenser tube and a thermometer to the three-necked flask, and set the temperature to 40°C. Under nitrogen atmosphere, the monomer St (23.3296 g, 224 mmol), the ligand PMDETA (0.23 mL, 1.12 mmol), and the macroinitiator polystyrene chloride ball PS-Cl (1 g, 1.12 mmol) and reducing agent stannous octoate (0.36 mL, 1.12 mmol). The reaction temperature was set to 110 °C and reacted for 9 h. After the reaction was completed, the reaction solution was poured into a beaker, allowed to stand for 12 h, filtered to remove the supernatant, and vacuum-dried to constant weight. The resulting product is the homopolymer brush PS-PSt.
[0039] (2) Add the cataly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com