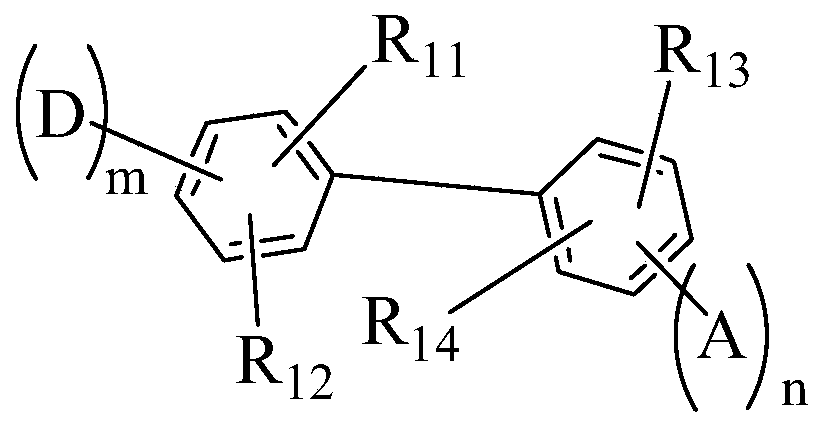
Compound, display panel and display device
A technology of compounds and derivatives, applied in the field of organic electroluminescence materials, to achieve the effects of reducing positive solvation color change effect, improving color purity, and low half-peak width
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Synthesis of compound H001
[0083]
[0084] S1 (10.0mmol), 9,9-dimethyl-9,10-dihydroacridine S2 (10.5mmol), (dibenzylideneacetone) dipalladium (0) (0.5mmol), sodium tert-butoxide (14mmol), tri-tert-butylphosphine tetrafluoroborate (1.0mmol) were put into a 500mL three-necked flask, while stirring, degassing and nitrogen replacement were repeated 3 times rapidly, and 200mL toluene was added through a syringe. The mixture was heated to reflux for 3 hours under nitrogen flow. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain Intermediate S3) (6.5 mmol, 65%).
[0085] MALDI-TOF MS: m / z, Calculated: C 27 h 22 BrN: 439.1; Test value: 439.4.
[0086]
[0087] At -78°C, S3 (1.5 mmol) was dissolved in ether (40 mL), and n...
Embodiment 2
[0090] Synthesis of compound H004
[0091]
[0092]At -78°C, S5 (2.5 mmol) was dissolved in ether (120 mL), and n-BuLi (3.5 mmol) in n-hexane was added dropwise to the solution. The reaction solution was continuously stirred for 2 h, slowly warmed to room temperature, and stirred at room temperature for 1 h. The reaction solution was cooled to -78°C again, and 45 mL of S4 (3.0 mmol) in toluene was added dropwise with stirring. Slowly warm to room temperature and stir overnight. All the solvent was distilled off under reduced pressure, and the crude product was collected. The crude product was washed with methanol (3 x 60 mL) and pentane (3 x 60 mL), respectively, and the crude product was collected again. The crude product was purified by silica gel column chromatography with n-hexane:chloroform (5:1) as eluent, and finally purified to give solid S6 (1.73 mmol, 69%).
[0093] Characterization results: MALDI-TOF MS: m / z, calculated value: C 32 h 19 B 2 FN 2 : 472.2; ...
Embodiment 3
[0099] Synthesis of compound H005
[0100]
[0101] At -78°C, S5 (2.0 mmol) was dissolved in ether (120 mL), and n-BuLi (2.5 mmol) in n-hexane was added dropwise to the solution. The reaction solution was continuously stirred for 2 h, slowly warmed to room temperature, and stirred at room temperature for 1 h. The reaction solution was cooled to -78°C again, and 45 mL of a toluene solution of S6 (2.4 mmol) was added dropwise with stirring. Slowly warm to room temperature and stir overnight. All the solvent was distilled off under reduced pressure, and the crude product was collected. The crude product was washed with methanol (3 x 50 mL) and pentane (3 x 50 mL), respectively, and the crude product was collected again. The crude product was purified by silica gel column chromatography with n-hexane:chloroform (5:1) as eluent, and finally purified to give solid S7 (3.25 mmol, 65%).
[0102] Characterization results: MALDI-TOF MS: m / z, calculated value: C 32 h 19 B 2 FN ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com