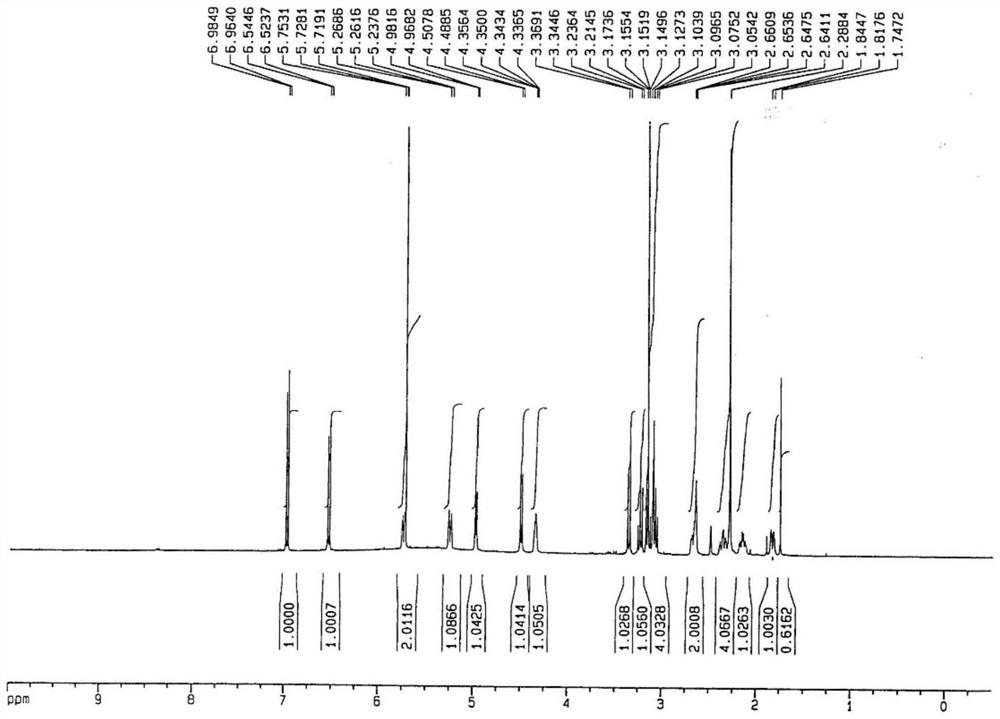
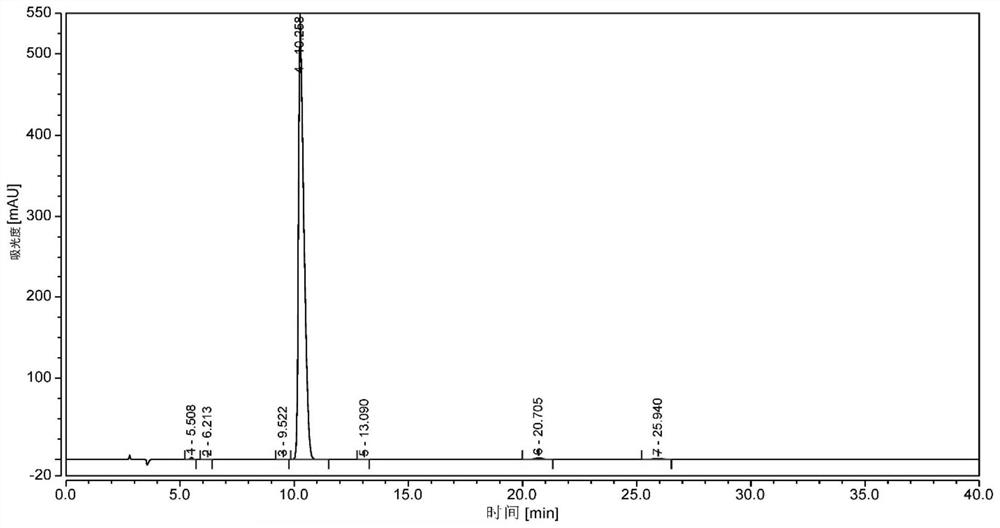
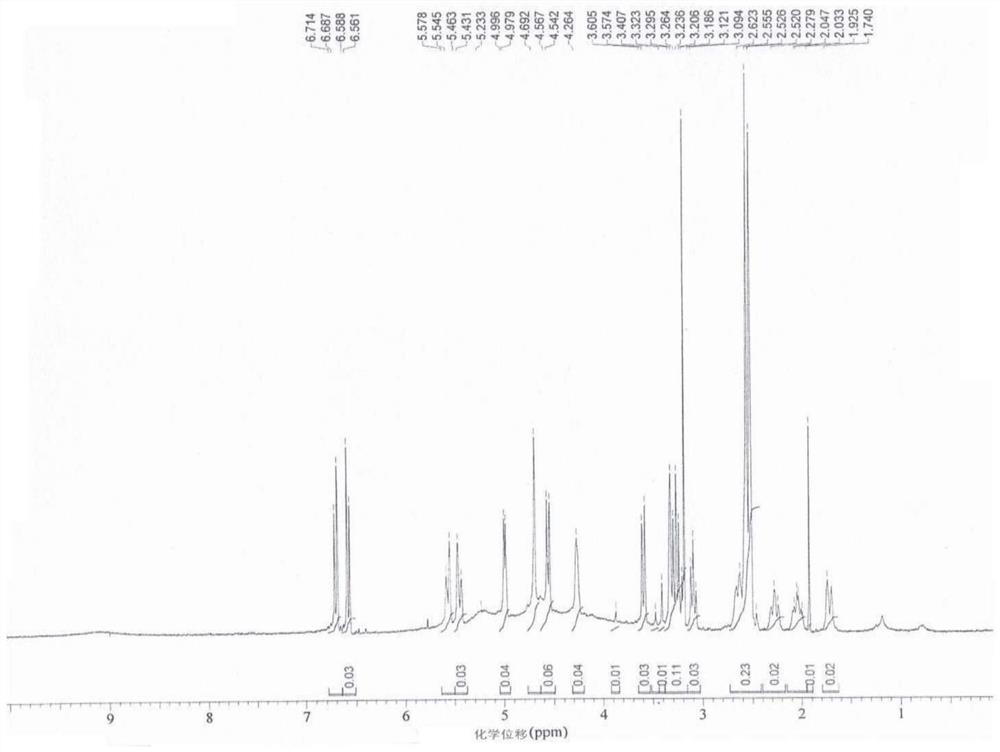
A kind of synthetic method of 10-carbonyl/hydroxymorphine-6-glucuronide
A glucuronide and synthesis method technology, applied in the field of organic synthesis, can solve the problems of unpublished preparation methods and structural identification information, and no generation of 10-carbonyl/hydroxymorphine-6-glucuronide, etc., achieving simple synthesis method, The effect of high purity and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0014] The present invention provides a kind of synthetic method of 10-carbonylmorphine-6-glucuronide, comprising:
[0015] 1) oxidizing the compound of formula (III) to obtain the compound of formula (IV),
[0016]
[0017] Wherein, R is formula (R-1) or formula (R-2),
[0018]
[0019] According to the present invention, the present invention oxidizes the compound of formula (III) structure to obtain the compound of formula (IV) structure, and the oxidizing agent of described oxidation is preferably one or both in chromium trioxide and manganese dioxide, and described Oxidation also includes oxidation aid, and described oxidation aid is trifluoroacetic acid or trifluoromethanesulfonic acid; The solvent of described reaction is methylene chloride or chloroform; The temperature of described reaction is 0~30 ℃, more preferably 10 ~25°C; in order to better carry out the reaction, the present invention preferably reacts at 5-10°C for 1-1.5 hours, and then reacts at 20-25°C...
Embodiment 1
[0049] Preparation of compound shown in embodiment 1 formula (I)
[0050]1) Preparation of compound shown in formula (III)
[0051] Take 14.1g of the dry compound represented by formula (II), dissolve it in 100ml of anhydrous chloroform, add 20g of potassium bicarbonate, add 28g of methyl chloroformate dropwise at room temperature, after the addition is complete, the reaction is completed at 55-60°C and cooled to room temperature , filtered, the filter cake was washed with 100ml chloroform, drained, combined the filtrates, concentrated to dryness, 15ml of ethyl acetate was recrystallized, crystallized at 0-5°C for 16-20h, suction filtered, the filter cake was washed with a small amount of glacial ethyl acetate, Dry at 40-45°C for 6-10 hours to obtain 8.32 g of the compound represented by formula (III). Yield: 55.5%
[0052] 2) Preparation of compound shown in formula (IV)
[0053] Take 7.63g of the compound represented by formula (III), dissolve it in 100ml of dichlorometha...
Embodiment 2
[0062] Preparation of compound shown in embodiment 2 formula (I)
[0063] 1) Preparation of compound shown in formula (III)
[0064] Take 14.1 g of the dry compound represented by formula (II), dissolve it in 100 ml of anhydrous dichloromethane, add 20 g of sodium bicarbonate, add 29.5 g of ethyl chloroformate dropwise at room temperature, and after the addition is complete, the reaction is completed at 40-45°C. Cool to room temperature, filter, wash the filter cake with 100ml of dichloromethane, drain it, combine the filtrates, concentrate to dryness, recrystallize in 15ml of ethyl acetate, crystallize at 0-5°C for 16-20h, suction filter, wash the filter cake with a small amount of ice Wash with ethyl acetate and dry at 40-45°C for 6-10 hours to obtain 8.65 g of the compound represented by formula (III). Yield: 56.6%.
[0065] 2) Preparation of compound shown in formula (IV)
[0066] Take 7.75g of the compound represented by formula (III), dissolve it in 100ml of dichlorom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com