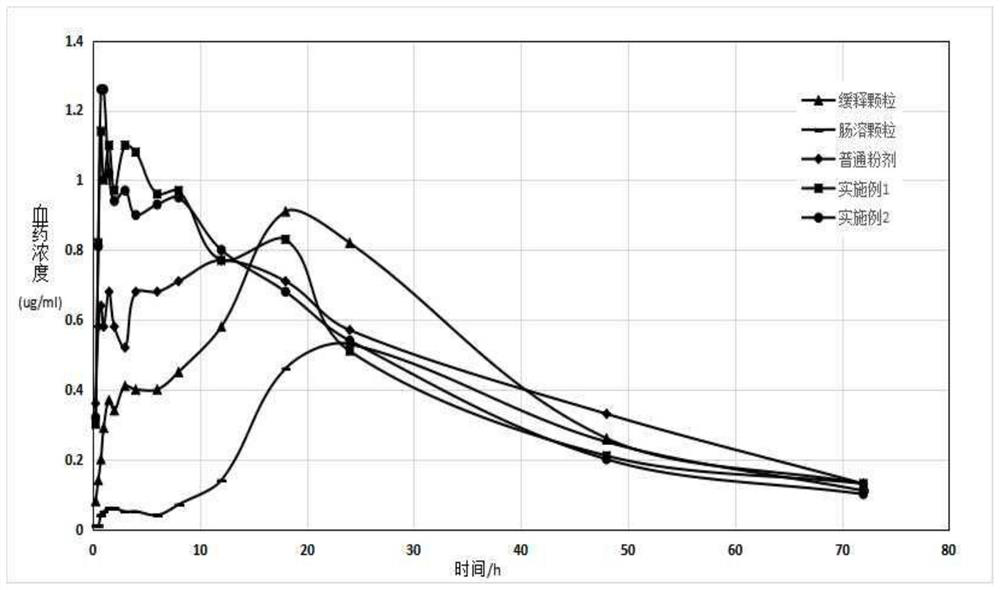
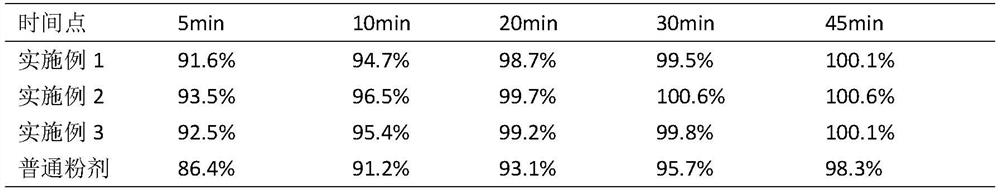
A kind of enrofloxacin quick-release pellets and preparation method thereof
A technology of enrofloxacin and immediate release, which is applied in the field of enrofloxacin immediate-release pellets and its preparation, can solve the problems of poor oral palatability of enrofloxacin powder, increase absorption and utilization time, expand production capacity, and achieve The effect of short peak times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Table 1 10% content specification prescription
[0034] Name of raw material Addition g Enrofloxacin 10g (converted content) dextrin 2g Croscarmellose Sodium 2g sucrose 10g starch added to 90g 2% pvpk30 aqueous solution 60ml 10% hypromellose solution 50ml Titanium dioxide 2.0g polyethylene glycol 400 3.0g water 80ml
[0035] Preparation process
[0036] (1) Take enrofloxacin, dextrin, sucrose, starch and cross-linked sodium carboxymethyl cellulose and mix uniformly by prescription quantity;
[0037] (2) Weigh the pvpk30 component and throw it into purified water, and dissolve it by shearing for 15 minutes to obtain a binder solution;
[0038] (3) Put the powder in step 1 into the tank mixer machine, mix with the binder in step 2 to prepare soft material, and discharge;
[0039] (4) Add the soft material prepared in step 3 to the spherical extruder and spheronizer for 35 rotations and extrude,...
Embodiment 2
[0044] Table 2 20% content specification prescription
[0045] Name of raw material Addition g Enrofloxacin Hydrochloride 20g (converted content) lactose 40g Crospovidone 3g starch added to 95g water 70ml Acrylic resin emulsion (gastric disintegration) 2 silica 2.0g Triacetin 1.0g ethanol 80ml
[0046] Preparation process
[0047] (1) Take enrofloxacin hydrochloride, crospovidone, lactose and starch by prescription and mix evenly;
[0048] (2) Put the powder in step 1 into the tank mixer machine, add water and mix to prepare soft material, and discharge;
[0049] (3) Add the soft material prepared in step 2 to a spherical extruder for 45 rotations to extrude, and spheronize in a spheronizer at 600 rotations for 60 seconds, and dry the prepared ball cores in a fluidized bed at 60 degrees for 1 hour;
[0050] (4) Screen the dried material, get the material between 20 and 60 mesh, and set aside;
[0051] (5...
Embodiment 3
[0054] Table 3 10% content specification prescription
[0055] Name of raw material Addition g Enrofloxacin Hydrochloride 5g (converted content) Enrofloxacin 5 (converted content) microcrystalline cellulose 40g Low Substituted Hydroxypropyl Methyl Cellulose 3g starch added to 90g water 70ml Acrylic resin emulsion (gastric disintegration) 7g talcum powder 2.0g triethyl citrate 1.0g ethanol 80ml
[0056] Preparation process
[0057] (1) Take enrofloxacin, enrofloxacin hydrochloride, low-substituted hypromellose, microcrystalline cellulose and starch by weighing and mixing uniformly according to the prescription quantity;
[0058] (2) Put the powder in step 1 into the tank mixer machine, add water and mix to prepare soft material, and discharge;
[0059] (3) Add the soft material prepared in step 2 to a spherical extruder for 45 rotations to extrude, and spheronize in a spheronizer at 600 rotations for 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com