Lithium perchlorate electrolyte solution and preparation method thereof
An electrolyte solution and lithium perchlorate technology, applied in the field of lithium ion batteries, can solve the problems of low solubility of lithium carbonate, difficult filtration, large equipment investment, etc., and achieve the effect of reducing the solid drying process, the preparation method is simple, and the degree of danger is reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
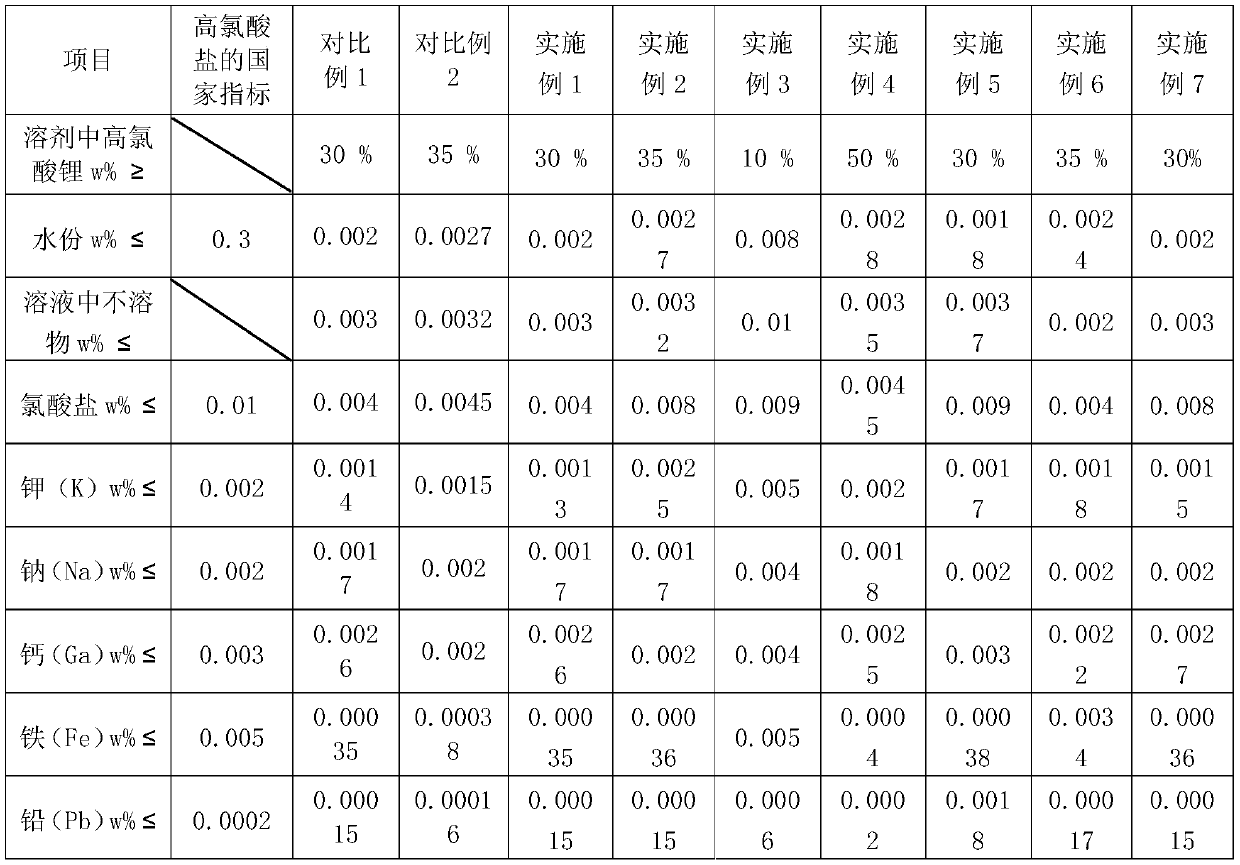
[0029] A preparation method of lithium perchlorate electrolyte solution, comprising the steps of: adding 50g of lithium chloride and 80g of sodium perchlorate into a reaction kettle, then adding 182mL of ethylene glycol dimethyl ether solution, the reaction temperature is 100°C, and stirring 3h, feed the inert gas argon to remove excess water, filter to remove the sodium chloride by-product insoluble in ethylene glycol dimethyl ether, evaporate the ethylene glycol dimethyl ether solvent, and return to the reaction kettle through reflux condensation, and the filtrate is again Return to the reaction kettle for secondary reaction until no precipitation occurs, heat the obtained filtrate to 80°C, then cool to 0°C and filter to remove unreacted sodium perchlorate, then filter the filtrate through 4A molecular sieves to remove the solvent Impurities and moisture in the solution, the lithium perchlorate finally obtained accounts for 30% of the mass percent content of the ethylene glyc...
Embodiment 2
[0032] A preparation method of lithium perchlorate electrolyte solution, comprising the steps of: adding 50g of lithium chloride and 80g of sodium perchlorate into a reaction kettle, then adding 182mL of diethyl carbonate solution, the reaction temperature is 140°C, stirring for 3h, Pass in the inert gas argon to remove excess water, filter to remove the sodium chloride by-product insoluble in diethyl carbonate, evaporate the diethyl carbonate solvent, return to the reaction kettle through reflux condensation, and return the filtrate to the reaction kettle again for secondary Reaction until no precipitation occurs, heat the obtained filtrate to 80°C, then cool to 0°C and filter to remove unreacted sodium perchlorate, then filter the filtrate through 4A molecular sieves to remove impurities and water in the solvent , the finally obtained lithium perchlorate accounts for 35% of the mass percentage of dimethyl carbonate solution, and the contents such as moisture and impurities in...
Embodiment 3
[0035] A preparation method of lithium perchlorate electrolyte solution, comprising the steps of: adding 60g of lithium bromide and 96g of sodium perchlorate into a reaction kettle, then adding 218mL of ethylene glycol dimethyl ether solution, the reaction temperature is 100°C, stirring for 3h, Pass in inert gas argon to remove excess water, lower the temperature to 51°C, filter out the precipitated sodium bromide by-product, evaporate the ethylene glycol dimethyl ether solvent, return to the reaction kettle after reflux condensation, and return the filtrate to the reaction vessel again Carry out a secondary reaction in the kettle until no precipitation occurs, heat the obtained filtrate to 80°C, then cool to 0°C and filter to remove unreacted sodium perchlorate, then filter the filtrate through 4A molecular sieves to remove Impurities and moisture, finally obtained lithium perchlorate accounts for 10% of the mass percent content of ethylene glycol dimethyl ether solution, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com