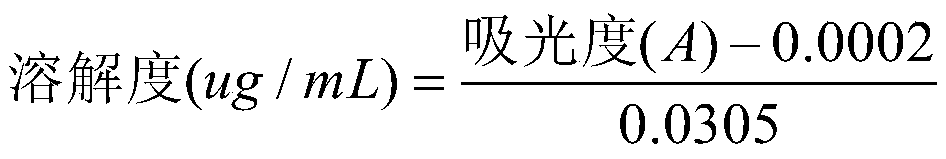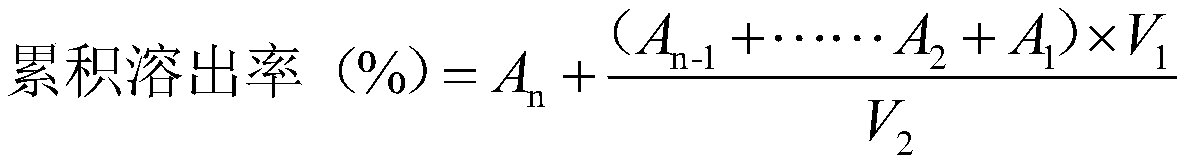Preparation method of chlorogenic acid lipid nanoparticle solid dispersion
A technology of solid dispersion and lipid nanoparticles, which can be used in medical preparations containing active ingredients, pharmaceutical formulations, organic active ingredients, etc., and can solve the problems of low drug percentage and large carrier amount.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
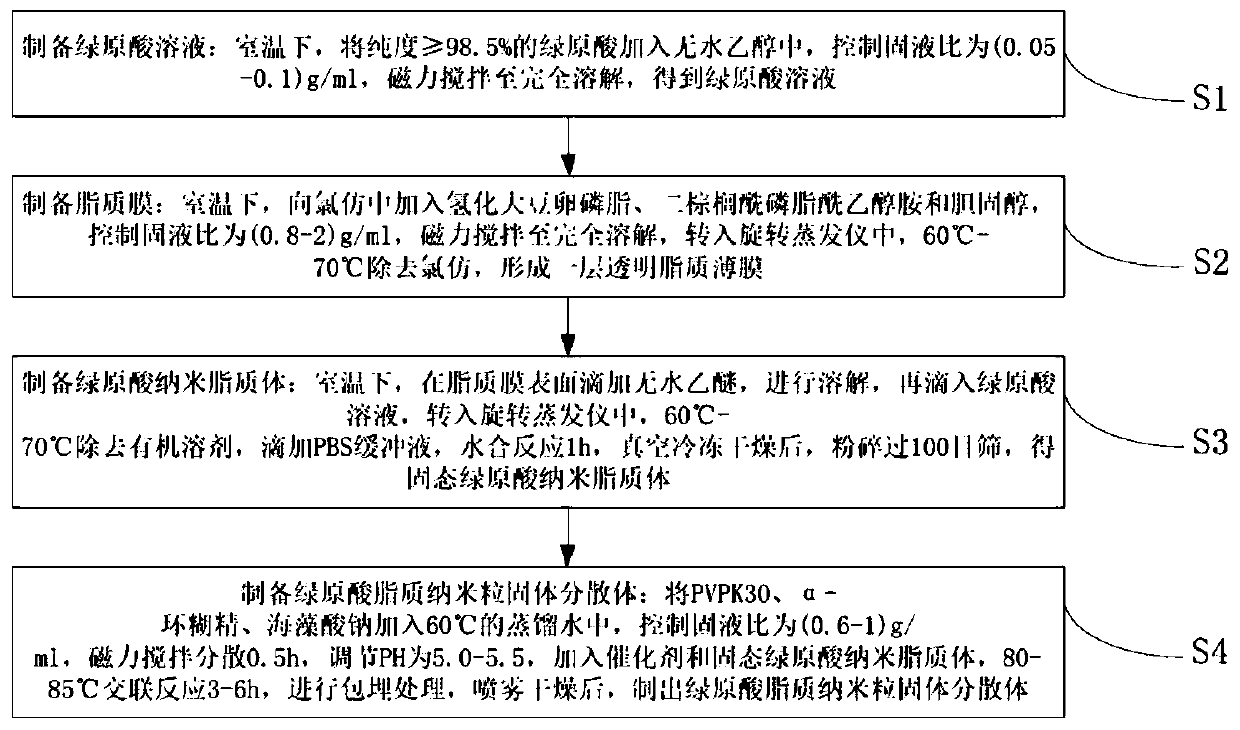
[0021] Embodiment 1 prepares chlorogenic acid lipid nanoparticle solid dispersion
[0022] S1: Preparation of chlorogenic acid solution: Weigh 2 g of chlorogenic acid with a purity ≥ 98.5%, add it to 40 ml of absolute ethanol at room temperature, control the solid-liquid ratio to 0.05 g / ml, stir magnetically until completely dissolved, and obtain chlorogenic acid acid solution;
[0023] S2: Preparation of lipid film: at room temperature, add 6g of hydrogenated soybean lecithin, 2g of dipalmitoylphosphatidylethanolamine and 2g of cholesterol to 12.5ml of chloroform, control the solid-liquid ratio to 0.8g / ml, magnetically stir until completely dissolved, turn Into a rotary evaporator, chloroform was removed at 60°C to form a transparent lipid film;
[0024] S3: Preparation of chlorogenic acid nanoliposomes: at room temperature, drop 6.4ml of anhydrous ether on the surface of the lipid film to dissolve, then drop into the chlorogenic acid solution, transfer to a rotary evaporato...
Embodiment 2
[0026] Embodiment 2 prepares chlorogenic acid lipid nanoparticle solid dispersion
[0027] S1: Preparation of chlorogenic acid solution: Weigh 2 g of chlorogenic acid with a purity ≥ 98.5%, add it to 20 ml of absolute ethanol at room temperature, control the solid-liquid ratio to 0.1 g / ml, stir magnetically until completely dissolved, and obtain chlorogenic acid acid solution;
[0028] S2: Preparation of lipid film: at room temperature, add 14g of hydrogenated soybean lecithin, 10g of dipalmitoylphosphatidylethanolamine and 5g of cholesterol to 14.5ml of chloroform, control the solid-liquid ratio to 2g / ml, magnetically stir until completely dissolved, transfer to In a rotary evaporator, remove chloroform at 70°C to form a transparent lipid film;
[0029] S3: Preparation of chlorogenic acid nanoliposomes: at room temperature, drop 17.4ml of anhydrous ether on the surface of the lipid film to dissolve, then drop into the chlorogenic acid solution, transfer to a rotary evaporato...
Embodiment 3
[0032] Measure the solubility of the chlorogenic acid lipid nanoparticles solid dispersion prepared by Example 1-2:
[0033] Weigh 1 mg of chlorogenic acid with a purity of ≥98.5% with an electronic balance, and weigh the corresponding amount of chlorogenic acid lipid nanoparticle solid dispersion prepared in Example 1-2 according to the standard that the content of chlorogenic acid in the medicine is 1 mg , with 10mL of artificial gastric juice as the dissolution medium, add 1mg of chlorogenic acid respectively, and the solid dispersion of chlorogenic acid lipid nanoparticles (containing 1 mg of chlorogenic acid) prepared in Example 1-2 is placed in a 37°C constant temperature water bath , 0.45μm microporous membrane filter, measure the absorbance and calculate the solubility of different samples at 37°C. Solubility calculation formula:
[0034]
[0035] With the chlorogenic acid bulk drug as the control group, the chlorogenic acid lipid nanoparticle solid dispersion prep...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com