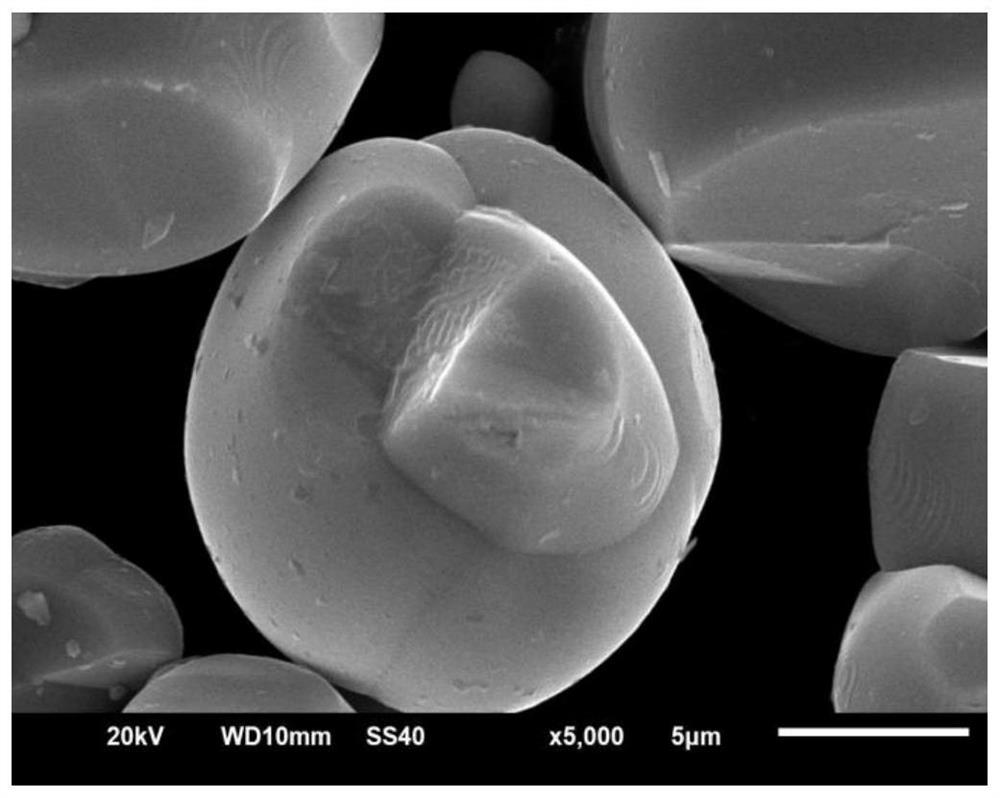
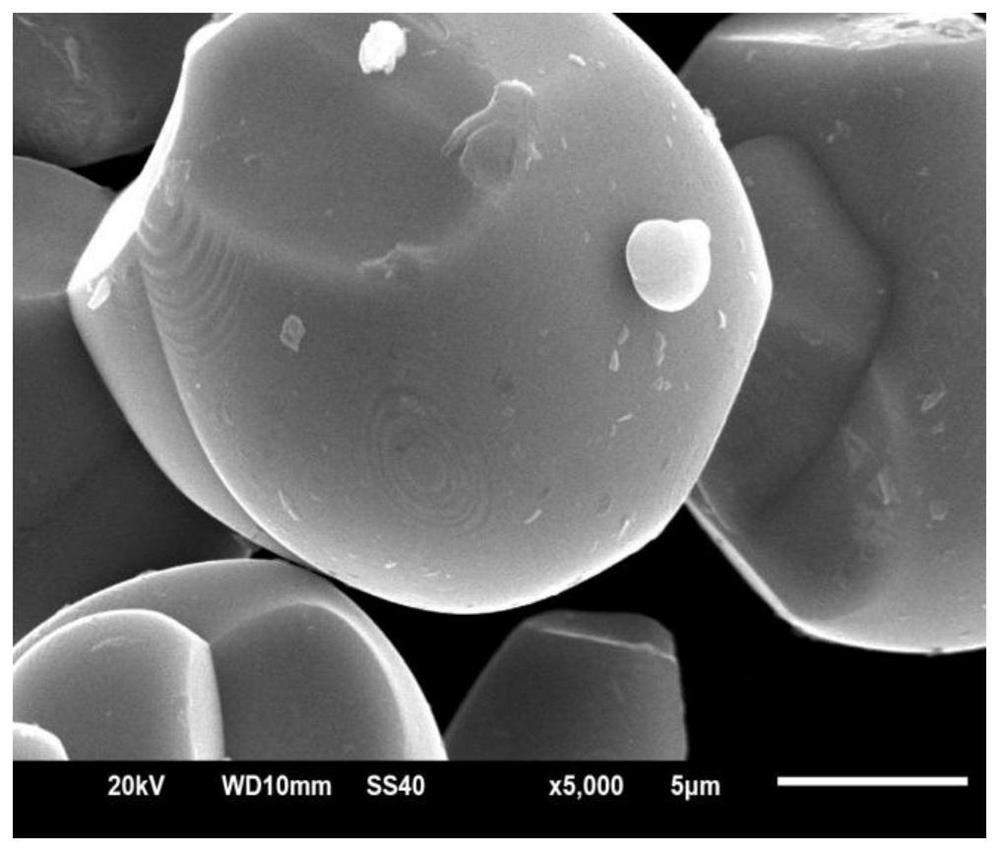
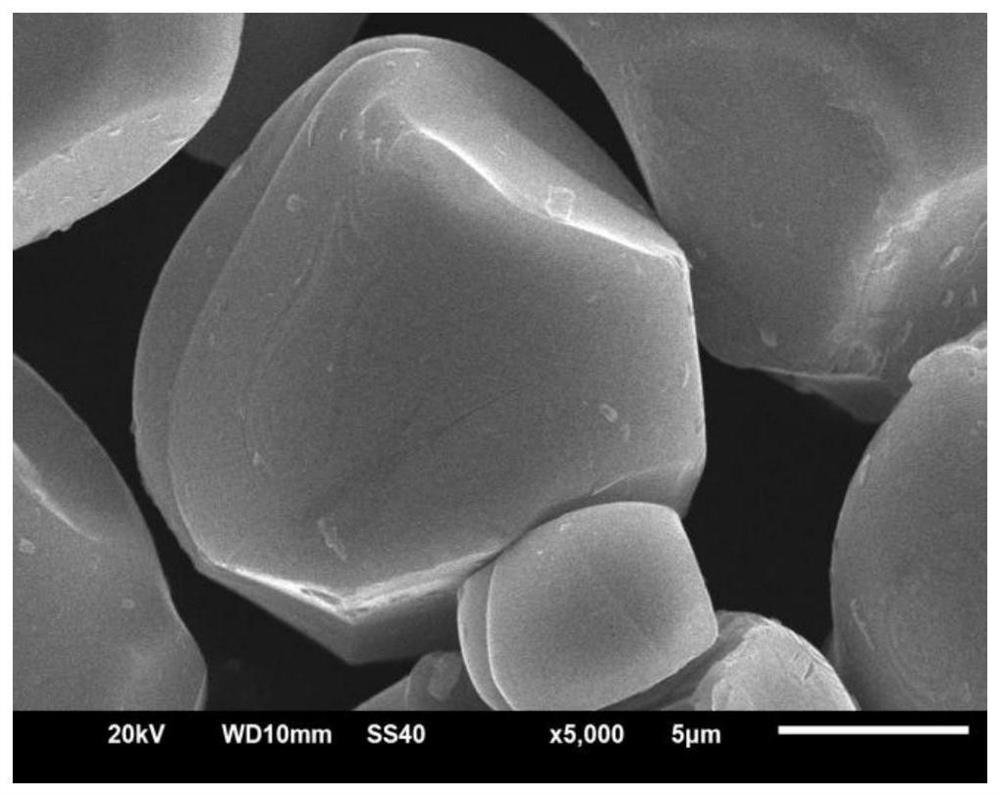
Lithium-ion battery lithium cobalt oxide cathode material and coating method thereof
A battery lithium cobalt oxide, positive electrode material technology, applied in the direction of battery electrodes, positive electrodes, secondary batteries, etc., can solve problems that are difficult to meet the requirements of practical applications, to improve cycle stability, reduce direct contact, and improve ion diffusion speed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] When the positive electrode material is prepared by a dry method for lithium ion battery lithium cobaltate positive electrode material, the preparation method comprises the following steps:
[0053] 1) Prepare the mixture precursor
[0054] In the present invention, the mixture precursor is prepared by fully mixing the lithium cobaltate particles and the reaction raw materials.
[0055] Wherein, the reaction raw materials include lithium salts or boron-containing compounds, stannous oxide or tin oxide, and ammonium dihydrogen phosphate, and the reaction raw materials generate tin-containing compounds on the surface of lithium cobaltate particles under high-temperature sintering conditions.
[0056] The lithium salt is selected from lithium carbonate, lithium hydroxide, Li 2 B 4 o 7 One or more of them, lithium carbonate is preferably used;
[0057] The boron-containing compound is selected from B 2 o 3 、H 3 BO 3 , Li 2 B 4 o 7 One or more of, preferably B 2 ...
Embodiment 1
[0088] Use a ball mill to pulverize the lithium carbonate, tin oxide and ammonium dihydrogen phosphate until the D90 is between 100-250nm, and then stop the pulverization.
[0089] Weigh lithium carbonate (Li 2 CO 3 )0.07g, tin oxide (SnO 2 )0.03g, ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ) 0.65g for mixing, after mixing evenly, add 100g of lithium cobaltate positive electrode and mix again to obtain the mixture precursor.
[0090] The above mixture precursor was placed in a tube furnace containing Ar protective gas, and sintered at a high temperature of 850° C. for 8 hours to prepare a lithium cobaltate cathode material coated with a tin-containing compound on the surface.
[0091] Wherein, the coating amount of the tin-containing compound on the surface of the lithium cobalt oxide positive electrode material is 1%.
Embodiment 2
[0093] The boron oxide, tin oxide and ammonium dihydrogen phosphate were respectively pulverized using a ball mill until the D90 was between 10-100 nm, and the pulverization was stopped.
[0094] Weigh tin oxide (SnO 2 )0.88g, boron oxide (B 2 o 3 )0.1g with NH 4 h 2 PO 4 0.35g was mixed, and after mixing evenly, 100g of lithium cobaltate cathode material was added and mixed again to obtain a mixture precursor.
[0095] Put the above mixture precursor in the H 2 The sintering reaction was carried out at a high temperature of 700° C. for 8 hours in a tube furnace with shielding gas to prepare a lithium cobalt oxide positive electrode material coated with a tin-containing compound on the surface.
[0096] Wherein, the coating amount of the tin-containing compound on the surface of the lithium cobalt oxide positive electrode material is 1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com