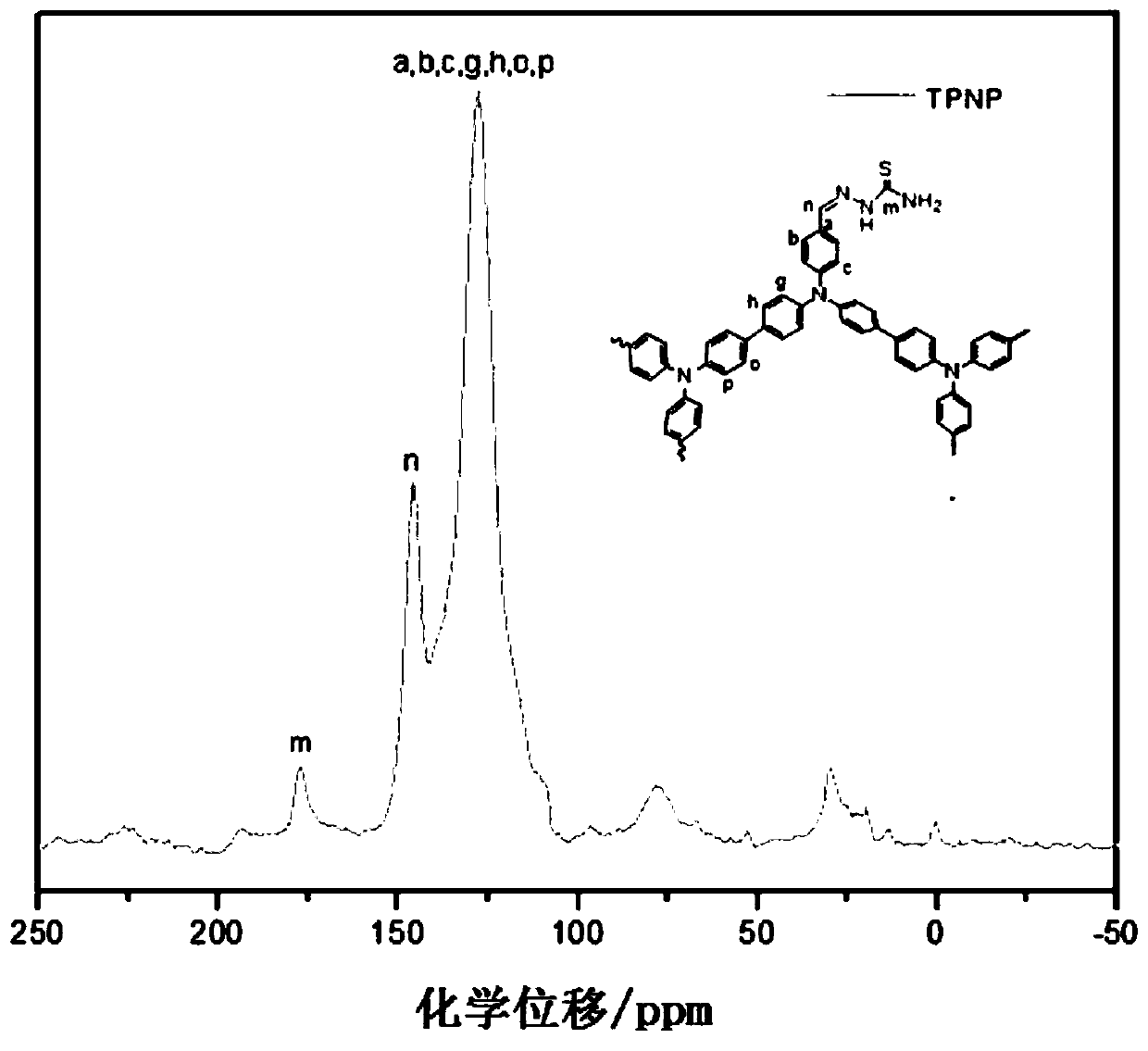
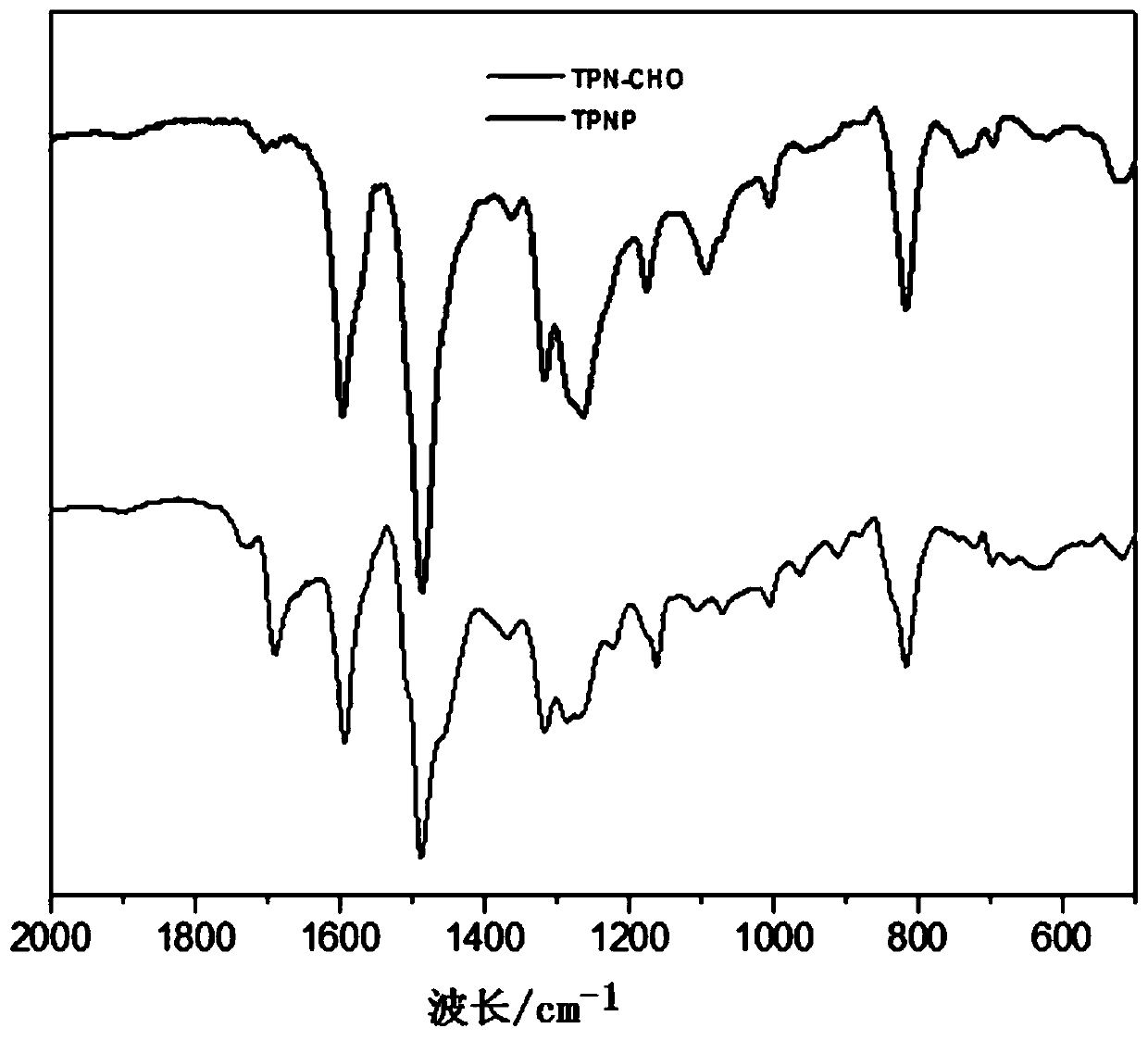
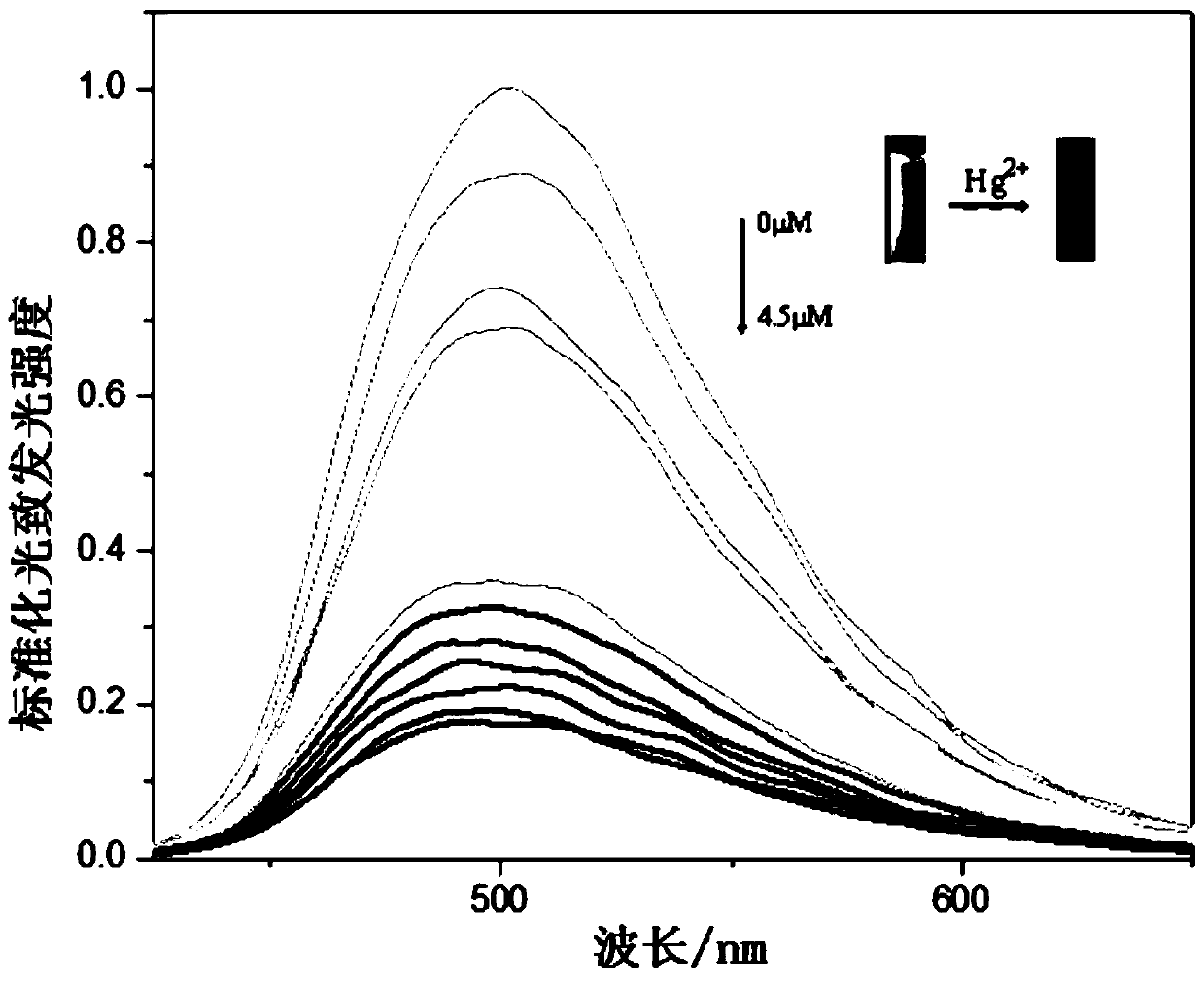
Macromolecular covalent organic framework polymer based on triphenylamine derivative, preparation method and application
A covalent organic framework and polymer technology, which is applied in the fields of material analysis, material excitation analysis, and material analysis through optical means, can solve the problems of polymers being difficult to disperse, difficult to realize sensing applications, and complex monomer synthesis, etc. Achieve the effects of sensitive removal, excellent photophysical properties, and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
[0049] 4-(diphenylamino)benzaldehyde (4g, 14.67mmol) was dissolved in 100mL of anhydrous tetrahydrofuran, and N-bromosuccinimide (5.2g, 29.34mmol) was added to the reaction flask, and the After reacting overnight at ℃, the solvent was removed by rotary evaporation under reduced pressure, and the target compound was purified by silica gel column chromatography. The eluent composition was petroleum ether: dichloromethane (v / v) = 4:1, and 5.79g of the product was obtained Compound 1, the yield was 92%; 1 H-NMR (400MHz, CDCl 3 -d): δ7.45–7.50(d,J58.8,4H),7.02–7.08(m,6H),7.73(d,J58.8,2H),9.86(s,1H). 13 CNMR (CDCl 3 ,400MHz,d):190.39,152.37,145.04,132.96,131.42,130.17,127.42,120.46,118.11.
[0050]
[0051] Three (4-bromophenyl) amine (4g, 8.35mmol), biboronic acid pinacol ester (8.5g, 33.4mmol) and potassium acetate (6.38g, 65mmol) were placed in a reaction flask, and under nitrogen atmosphere After deoxygenation under nitrogen for 15 minutes, [1,1'-bis(diphenylp...
Embodiment 2
[0059]
[0060] Disperse 100 mg of high molecular polymer with compound 3 as a repeating unit in 30 mL of ethanol, then add 2-acetylpyridine (157 mg, 1.30 mmol), potassium hydroxide (73 mg, 1.30 mmol), and 106 mg of ammonia water, and stir at room temperature React for 48 hours. After the reaction was completed, the solvent was removed by rotary evaporation under reduced pressure, the residue was washed with methanol, and the methanol was separated by centrifugation. The obtained solid was vacuum-dried at 65°C for 8 hours to obtain a polymeric covalent organic framework with compound 5 as the repeating unit Polymer, in the formula Indicates the linking position of the repeatable structural unit, compound 5 can be repeated 16 times.
Embodiment 3
[0062]
[0063] Disperse 100 mg of high molecular polymer with compound 3 as a repeating unit in 30 mL of ethanol, then add malononitrile (58 mg, 0.88 mmol), triethylamine (1 mL), and react under reflux at 80° C. for 24 hours. After the reaction, the solvent was removed by rotary evaporation under reduced pressure, the residue was washed with methanol, and the methanol was separated by centrifugation. The resulting solid was dried in vacuum at 65°C for 8 hours to obtain a polymeric covalent organic framework with compound 6 as the repeating unit Polymer, in the formula Indicates the linking position of the structural unit that can be repeated, compound 6 can be repeated 16 times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com