Macromolecular compound containing boron-nitrogen coordinate bond, preparation method and application thereof
A technology of polymer compounds and coordination bonds, which is used in semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problems of slow development, low mobility, poor air stability, etc. Good mobility and flatness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
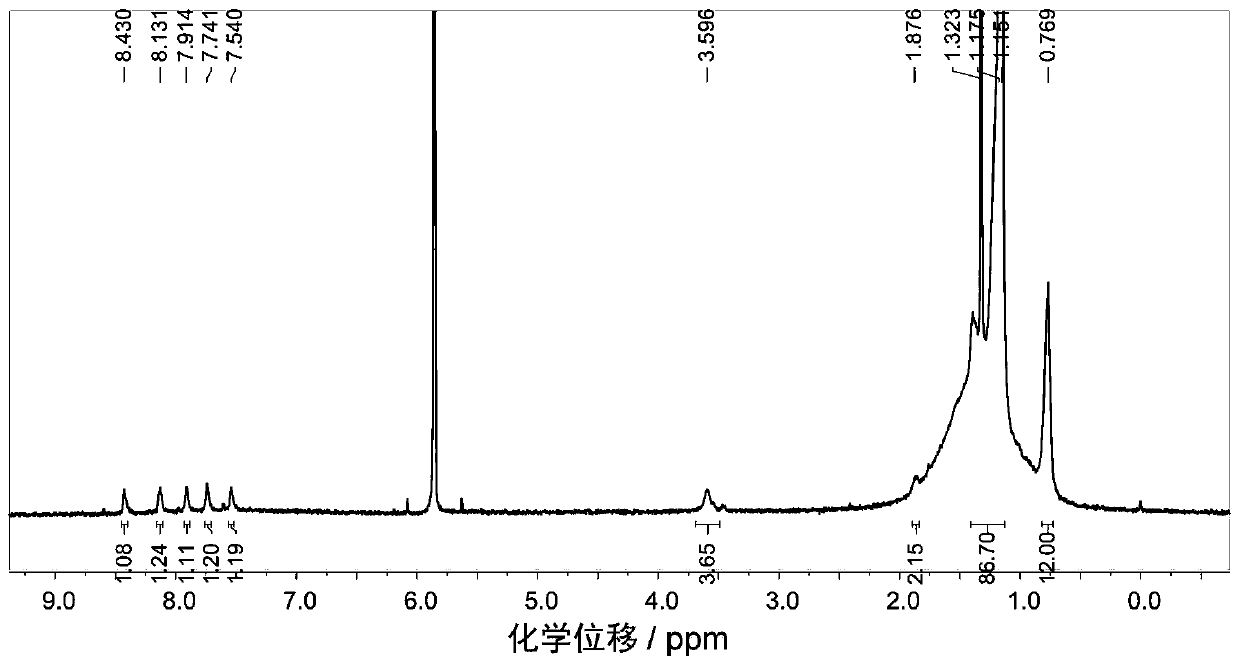
Embodiment 1
[0096] P-BNBP-DTBT (C24) polymer, the structural formula is as follows (in the structural formula, the capping group is omitted):
[0097]
[0098] The preparation method is: add BNBP dibromomonomer (111.3mg, 0.10mmol), thiophene bridged benzothiadiazole bistin salt (62.6mg, 0.10mmol) and tetrakis(triphenyl Phosphine) palladium (1.2mg, 0.001mmol), then vacuumize the system and ventilate the system with argon for several times, add distilled toluene solvent (15mL) in the dark state, reflux at 115°C for 48h, and then add benzene Boric acid (100mg, 0.82mmol) was continued to reflux for 3h, and bromobenzene (200mg, 1.28mmol) was added to reflux for 12h. The reaction system was settled in methanol while it was hot, and the polymer was precipitated. The precipitate was washed with acetone, n-hexane, and chloroform to remove small molecules and catalysts with a Soxhlet extractor, and finally the polymer was extracted with chlorobenzene. Yield 100.2 mg, 78% yield.
[0099] Elemen...
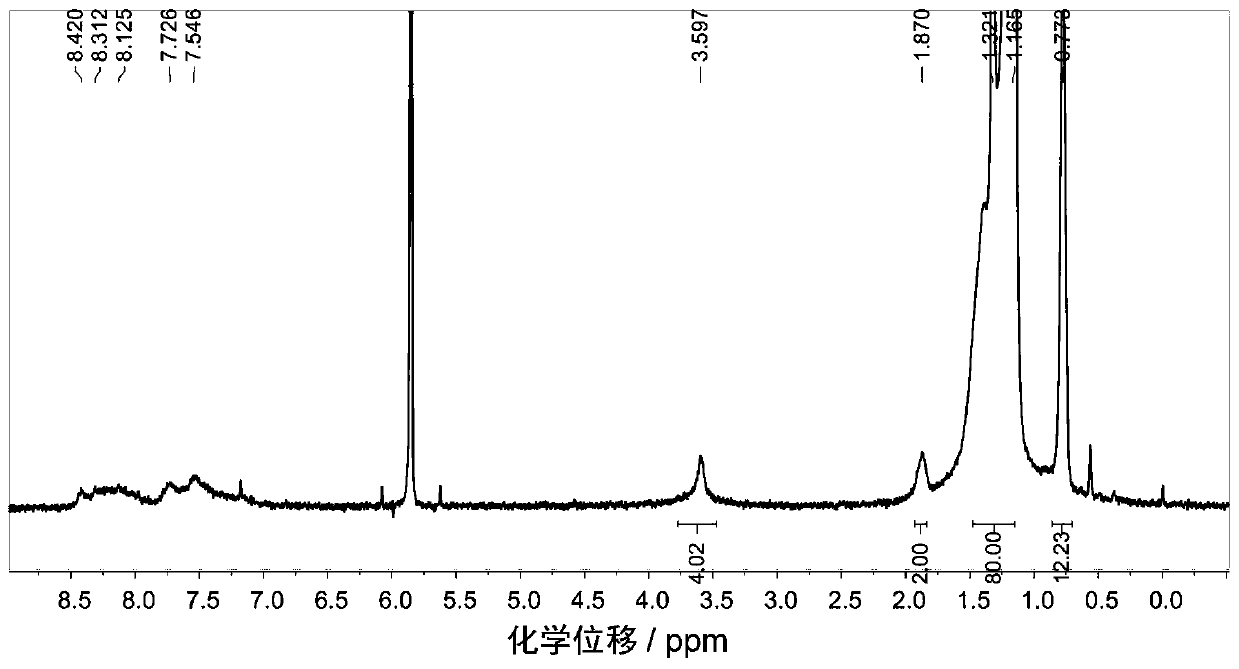
Embodiment 2
[0103] P-BNBP-DTBT (C28) polymer, the structural formula is as follows (in the structural formula, the capping group is omitted):
[0104]
[0105] The preparation method is: add BNBP dibromomonomer (122.5mg, 0.10mmol), thiophene bridged benzothiadiazole bistin salt (62.6mg, 0.10mmol) and tetrakis(triphenyl Phosphine) palladium (1.2mg, 0.001mmol), then vacuumize the system and ventilate the system several times with argon, add distilled toluene solvent (35mL) in a dark state, reflux at 115°C for 52h, then add phenylboronic acid (100mg, 0.82mmol) was refluxed for 3h, and bromobenzene (200mg, 1.28mmol) was added to reflux for 3h. The reaction system was settled in methanol while it was hot, and the polymer was precipitated. The precipitate was washed with acetone, n-hexane, and chloroform to remove small molecules and catalysts with a Soxhlet extractor, and finally the polymer was extracted with chlorobenzene. Yield 119 mg, 92% yield.
[0106] The elemental analysis of the ...
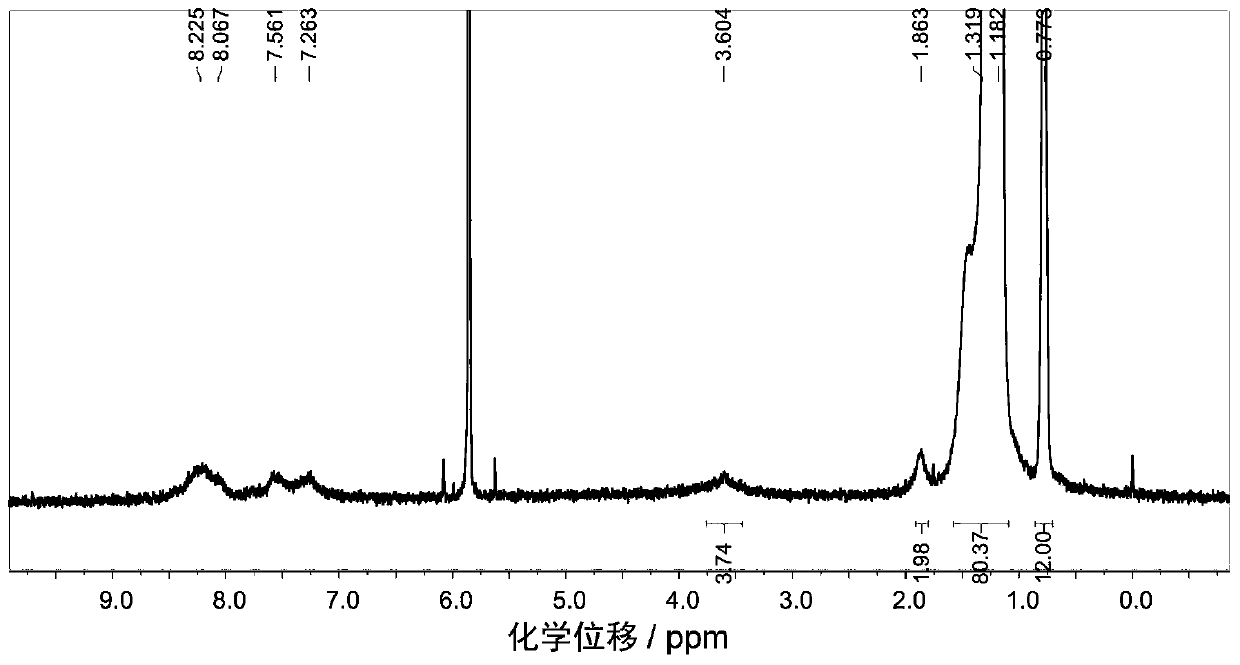
Embodiment 3
[0109] P-BNBP-DTBT (C32) polymer, the structural formula is as follows (in the structural formula, the capping group is omitted):
[0110]
[0111] The preparation method is: add BNBP dibromomonomer (133.7mg, 0.10mmol), thiophene bridged benzothiadiazole bistin salt (62.6mg, 0.10mmol) and tetrakis (triphenyl Phosphine) palladium (1.2mg, 0.001mmol), then vacuumize the system and ventilate the system several times with argon gas, add distilled toluene solvent (15mL) in a dark state, reflux at 115°C for 48h, then add phenylboronic acid (100mg, 0.82mmol) was refluxed for 3h, and bromobenzene (200mg, 1.28mmol) was added to reflux for 3h. The reaction system was settled in methanol while it was hot, and the polymer was precipitated. The precipitate was washed with acetone, n-hexane, and chloroform to remove small molecules and catalysts with a Soxhlet extractor, and finally the polymer was extracted with chlorobenzene. Yield 110 mg, 74% yield.
[0112] Elemental analysis was ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electron mobility | aaaaa | aaaaa |
| Hole mobility | aaaaa | aaaaa |
| Electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com