Soluble thiophene copolymer with electron-withdrawing group and preparation method and application of soluble thiophene copolymer
An electron-withdrawing-based, copolymer technology, applied in circuits, photovoltaic power generation, electrical components, etc., can solve the problems of general solubility, limited application of P3HT, inferior to other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Preparation of monomethyl ether diethoxyethyl thiophene carboxylate (MECT)
[0145] The synthetic process is shown in route (1'):
[0146]
[0147] Among them, R 1 It is 2-(2-(2-methoxyethoxy)ethoxy)ethyl carboxylate, abbreviated as MPEG-3OOC, and the ester bond -COO- is a strong electron-withdrawing group.
[0148] The specific synthesis steps are as follows:
[0149] N 2 To a purified three-necked round-bottom flask, add 300 mL of redistilled dichloromethane (DCM), 5.19 g (40.5 mmol, 1.0 equivalents) of 3-thiophenecarboxylic acid (TCA), 2-(2-( 2-methoxyethoxy) ethoxy) ethanol (2-(2-(2-methoxyethoxy)ethoxy)ethanol) 6.65g (6.5mL, 40.5mmol, 1.0 equivalent) and esterification catalyst 4-dimethylaminopyridine (DMAP) 7.40 g (60.5 mmol, 1.5 equiv). The reactant was colorless, clear and transparent, and was stirred at room temperature for 1 h. then cooled to 0°C in N 2 9.19 g (44.5 mmol, 1.1 equivalents) of dicyclohexylcarbodiimide (DCC) was added under atmosphere. ...
Embodiment 2
[0153] Preparation of monomer 3-heptyloxythiophene (HPOT)
[0154] Synthesis is shown in route (2'):
[0155]
[0156] Among them, R 3 For heptyloxy.
[0157] The specific synthesis steps are as follows:
[0158] After adding CuI 11.20g (58.80mol, 0.20 equivalents), potassium tert-butoxide tBuOK49.60g (442.0mols, 1.50 equivalents), n-heptanol 83.24mL (588.8mols, 2.00 equivalents) in a 500mL round bottom flask, Pure N 2 The round bottom flask was purged and stirred at room temperature for 1 h. Add 27.60 mL (294.4 mol, 1.00 equivalent) of 3-bromothiophene (substance 1), reaction temperature: 100° C., reaction time: 24 h. Cool to room temperature after the reaction.
[0159] Refining method: the mixture was washed with tetrahydrofuran (THF), filtered to remove most of the inorganic salts, the filtrate was collected, and 44.44 g of a light yellow oily liquid product 3-heptyloxythiophene (HPOT) was obtained by distillation under reduced pressure, with a yield of 76.12%.
...
Embodiment 3
[0162] Preparation of poly(monomethyl ether diethoxyethylthiophene carboxylate / 3-hexylthiophene) P(MECT-3HT)
[0163] Synthesis is shown in route (3'):
[0164]
[0165]
[0166] Among them, R 1 It is 2-(2-(2-methoxyethoxy)ethoxy)ethyl carboxylate, abbreviated as MPEG-3OOC, and the ester bond -COO- is a strong electron-withdrawing group; R 2 For hexyl.
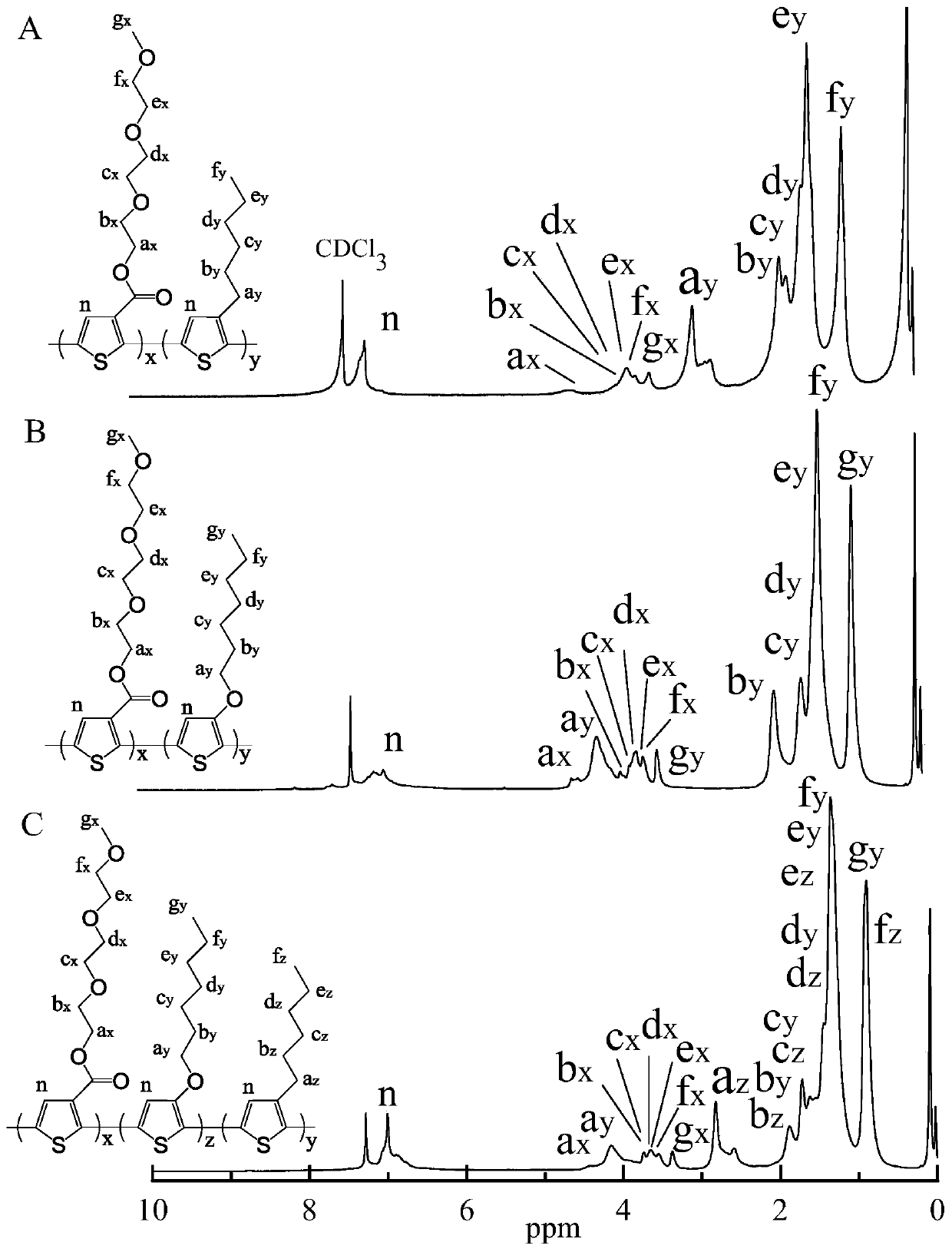
[0167] In this example, the prepared thiophene copolymer poly(monomethyl ether diethoxyethyl thiophene carboxylate / 3-hexylthiophene) P(MECT-3HT) has a thiophene chain as the core, and at its β-position Electron-withdrawing group MPEG-3OOC and hexyl side chain, the structural formula is shown in formula (A),
[0168]
[0169] The general formula is shown in the following formula (X):
[0170]
[0171] Among them, in formula (X), R 1 The ester bond is a strong electron-withdrawing group; a=3, b=5, c=6; R 4 =-CH 3 ; x=2.9, y=34.8, z=0.
[0172] Due to FeCl 3 If it is not removed, Fe will be generated 2 o 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
| Resistance | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com