A kind of soluble thiophene copolymer with electron-withdrawing group and its preparation method and application
An electron-withdrawing-based, copolymer technology, applied in circuits, photovoltaic power generation, electrical components, etc., can solve the problems of inferior, limited application of P3HT, general solubility, etc., achieve high efficiency, excellent hole transport performance, and improve air stability. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] Preparation of monomethyl ether diethoxyethyl thiophene carboxylate (MECT)
[0145] The synthetic process is shown in route (1'):
[0146]
[0147] Among them, R 1 It is 2-(2-(2-methoxyethoxy)ethoxy)ethyl carboxylate, abbreviated as MPEG-3OOC, and the ester bond -COO- is a strong electron-withdrawing group.
[0148] The specific synthesis steps are as follows:
[0149] N 2 To a purified three-necked round-bottom flask, add 300 mL of redistilled dichloromethane (DCM), 5.19 g (40.5 mmol, 1.0 equivalents) of 3-thiophenecarboxylic acid (TCA), 2-(2-( 2-methoxyethoxy) ethoxy) ethanol (2-(2-(2-methoxyethoxy)ethoxy)ethanol) 6.65g (6.5mL, 40.5mmol, 1.0 equivalent) and esterification catalyst 4-dimethylaminopyridine (DMAP) 7.40 g (60.5 mmol, 1.5 equiv). The reactant was colorless, clear and transparent, and was stirred at room temperature for 1 h. then cooled to 0°C in N 2 9.19 g (44.5 mmol, 1.1 equivalents) of dicyclohexylcarbodiimide (DCC) was added under atmosphere. ...
Embodiment 2
[0153] Preparation of monomer 3-heptyloxythiophene (HPOT)
[0154] Synthesis is shown in route (2'):
[0155]
[0156] Among them, R 3 For heptyloxy.
[0157] The specific synthesis steps are as follows:
[0158] After adding CuI 11.20g (58.80mol, 0.20 equivalents), potassium tert-butoxide tBuOK49.60g (442.0mols, 1.50 equivalents), n-heptanol 83.24mL (588.8mols, 2.00 equivalents) in a 500mL round bottom flask, Pure N 2 The round bottom flask was purged and stirred at room temperature for 1 h. Add 27.60 mL (294.4 mol, 1.00 equivalent) of 3-bromothiophene (substance 1), reaction temperature: 100° C., reaction time: 24 h. Cool to room temperature after the reaction.
[0159] Refining method: the mixture was washed with tetrahydrofuran (THF), filtered to remove most of the inorganic salts, the filtrate was collected, and 44.44 g of a light yellow oily liquid product 3-heptyloxythiophene (HPOT) was obtained by distillation under reduced pressure, with a yield of 76.12%.
...
Embodiment 3
[0162] Preparation of poly(monomethyl ether diethoxyethylthiophene carboxylate / 3-hexylthiophene) P(MECT-3HT)
[0163] Synthesis is shown in route (3'):
[0164]
[0165]
[0166] Among them, R 1 It is 2-(2-(2-methoxyethoxy)ethoxy)ethyl carboxylate, abbreviated as MPEG-3OOC, and the ester bond -COO- is a strong electron-withdrawing group; R 2 For hexyl.
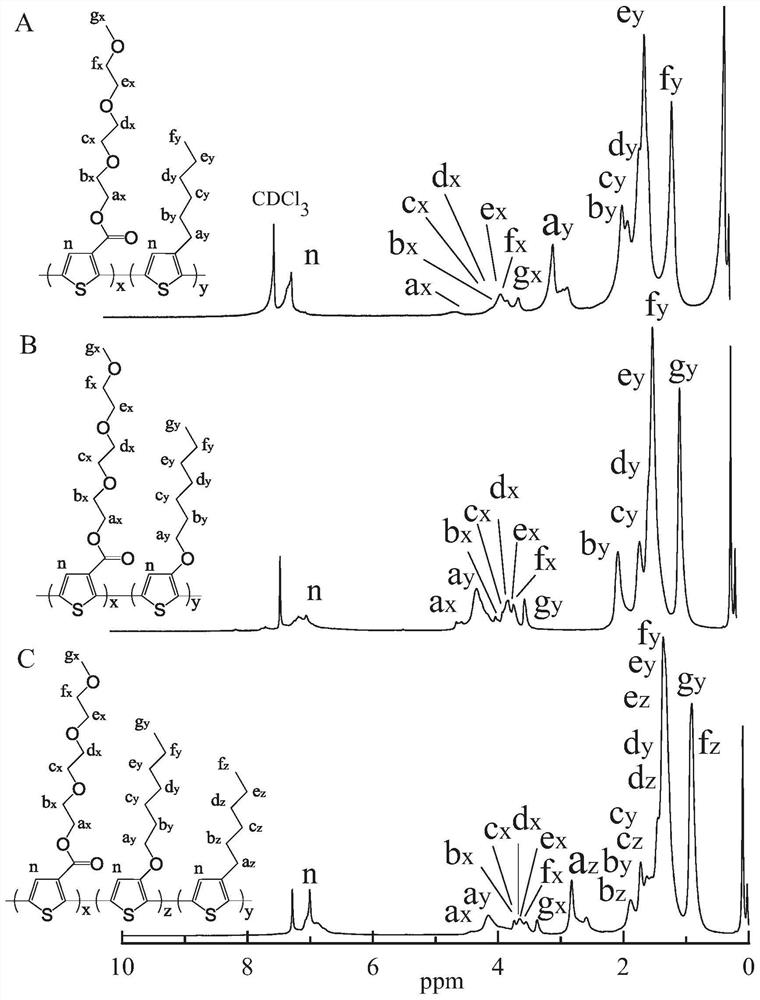
[0167] In this example, the prepared thiophene copolymer poly(monomethyl ether diethoxyethyl thiophene carboxylate / 3-hexylthiophene) P(MECT-3HT) has a thiophene chain as the core, and at its β-position Electron-withdrawing group MPEG-3OOC and hexyl side chain, the structural formula is shown in formula (A),
[0168]
[0169] The general formula is shown in the following formula (X):
[0170]
[0171] Among them, in formula (X), R 1 The ester bond is a strong electron-withdrawing group; a=3, b=5, c=6; R 4 =-CH 3 ; x=2.9, y=34.8, z=0.
[0172] Due to FeCl 3 If it is not removed, Fe will be generated 2 o 3 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com