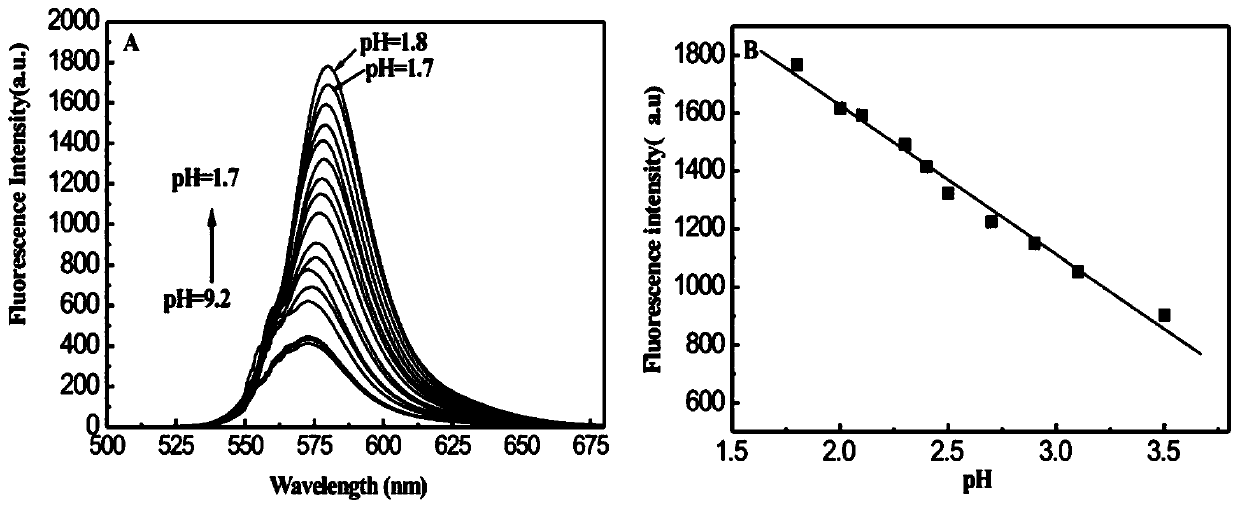
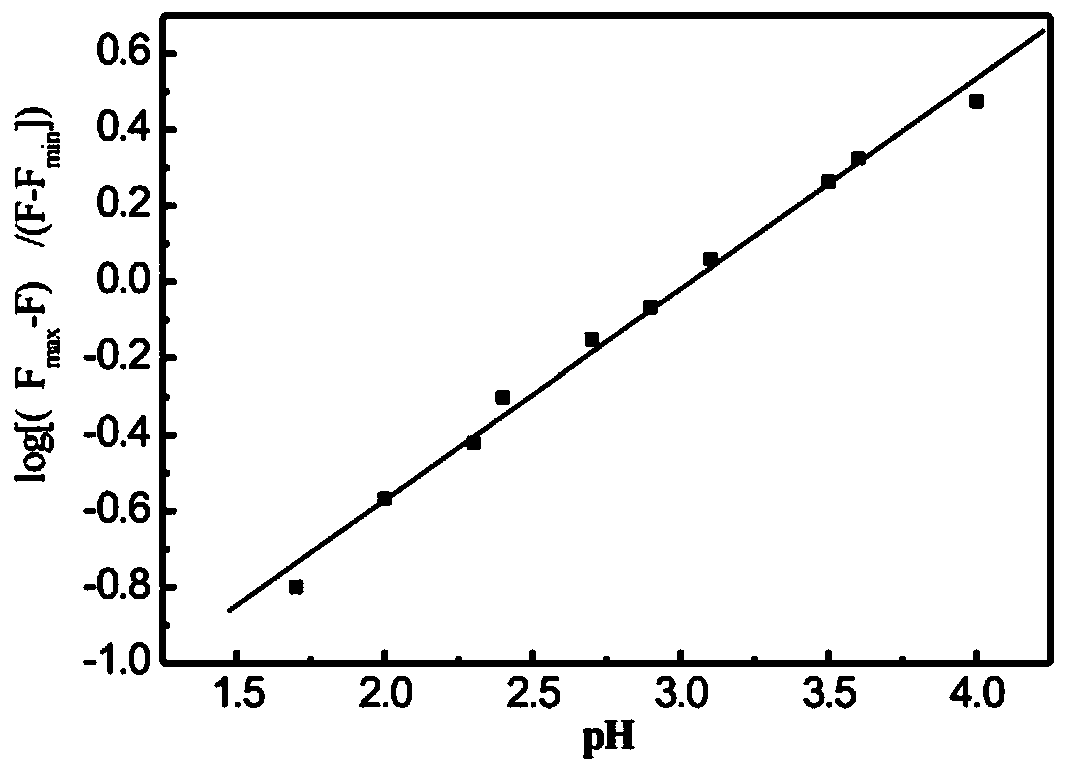
Preparation method and application of 1-methylpiperazine rhodamine amide
A technology of rhodamine amide and methylpiperazine is applied in the field of simple preparation of rhodamine B derivative optical sensing materials, and can solve the problems of complex hydrogen ion identification and detection methods, low identification response sensitivity, poor selectivity and the like , to achieve the effect of strong reversibility and stability, fast and sensitive optical response, and low consumption of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
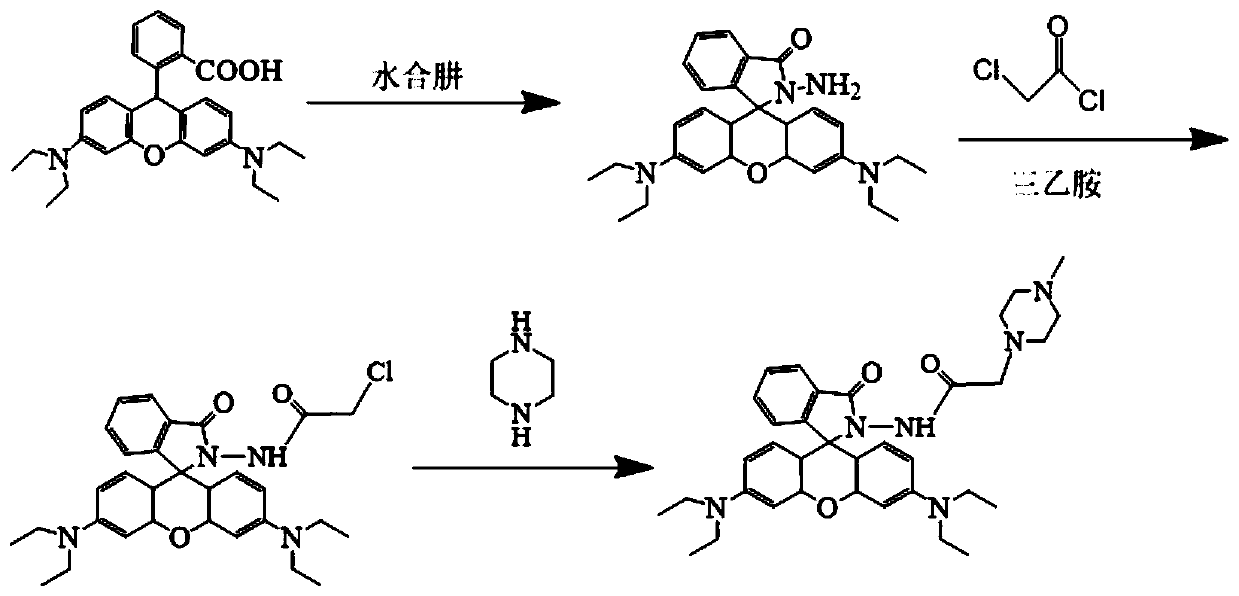
[0029] The present invention a kind of preparation method (as figure 1 shown), specifically comprising the following steps: firstly dissolving Rhodamine B in an appropriate amount of ethanol, adding hydrazine hydrate after stirring, heating to reflux and reducing the reaction for a certain period of time, then concentrating the solvent to obtain a yellow crude product, and then using concentrated hydrochloric acid and a certain concentration of Sodium hydroxide adjusts the acidity of the reaction medium to nearly neutral, and a large amount of precipitates are precipitated. After filtering and drying, a light pink rhodamine B hydrazide intermediate product powder is obtained; then rhodamine B hydrazide is dissolved in methylene chloride, and chloroacetyl chloride is added React with acid-binding agent triethylamine, stir rapidly under ice-salt bath, remove solvent, obtain purple solid crude product, and obtain white solid after neutral alumina passes through the column; finally...
Embodiment 1
[0032] (1) Dissolve Rhodamine B 1.2g (2.5mmol) in 30mL ethanol solvent, add 98% (mass percentage) hydrazine hydrate 2mL, heat and reflux at 80°C for 3h, concentrate the solvent, precipitate a yellow product, pump After filtering and drying, put it in a small beaker, continuously add concentrated hydrochloric acid dropwise to completely dissolve the product into a purple-red solution, then adjust the pH value of the system to about 7.0 with 40% aqueous sodium hydroxide solution, and precipitate a large amount of light pink solid, suction filter and dry Obtain light pink rhodamine B hydrazide intermediate product powder afterwards;
[0033] (2) Weigh 0.456g (1mmol) of rhodamine B hydrazide and dissolve it in 25mL of dichloromethane, add 0.4mL (2.50mmol) of chloroacetyl chloride, and add 0.5mL of triethylamine as an acid-binding agent, The reaction was rapidly stirred in an ice-salt bath for 10 h, and the solvent was evaporated to obtain a crude lavender solid product, which was ...
Embodiment 2
[0038](1) Dissolve Rhodamine B 1.2g (2.5mmol) in 30mL ethanol solvent, add 98% (mass percentage) hydrazine hydrate 2.5mL, heat and reflux at 90°C for 1.5 hours, concentrate the solvent, and precipitate a yellow product , suction filtered, dried and placed in a small beaker, continuously dropwise added concentrated hydrochloric acid to completely dissolve the product into a purple-red solution, then adjusted the pH value of the system to about 7.0 with 40% aqueous sodium hydroxide solution, a large amount of pale pink solid was precipitated, and suction filtered 1. Obtain light pink rhodamine B hydrazide intermediate product powder after drying;
[0039] (2) Weigh 0.456g (1mmol) of rhodamine B hydrazide and dissolve it in 25mL of dichloromethane, add 0.4mL (2.50mmol) of chloroacetyl chloride, and add 1.0mL of triethylamine as an acid-binding agent, The reaction was rapidly stirred in an ice-salt bath for 10 h, and the solvent was evaporated to obtain a crude lavender solid prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com