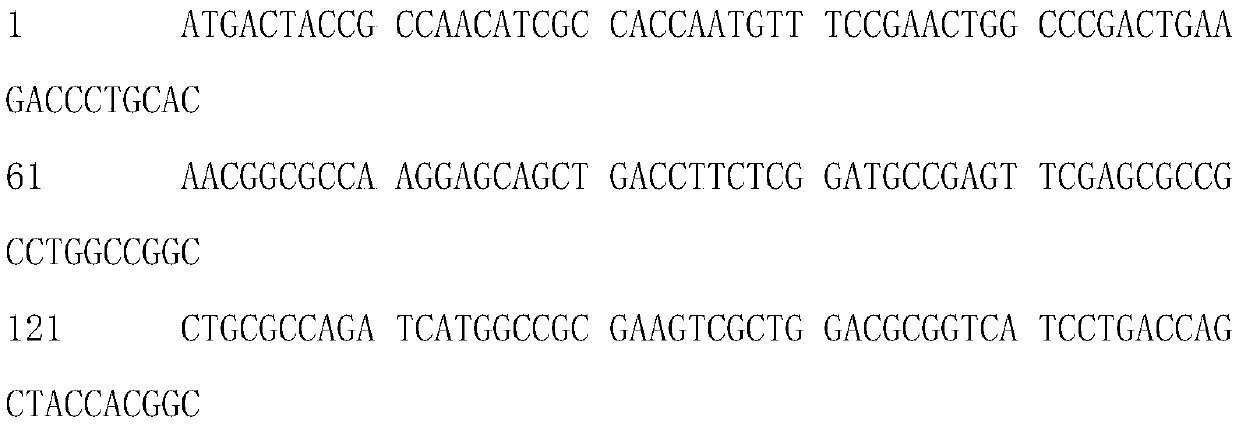A kind of improved highly active thermostable creatine hydrolase and its application
A technology of creatine hydrolase and amino acid, applied in the direction of hydrolase, application, enzyme, etc., can solve the problem of thermal stability not suitable for industrial production, and achieve the effect of improving thermal stability and specific activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Obtaining of highly active mutant sequences
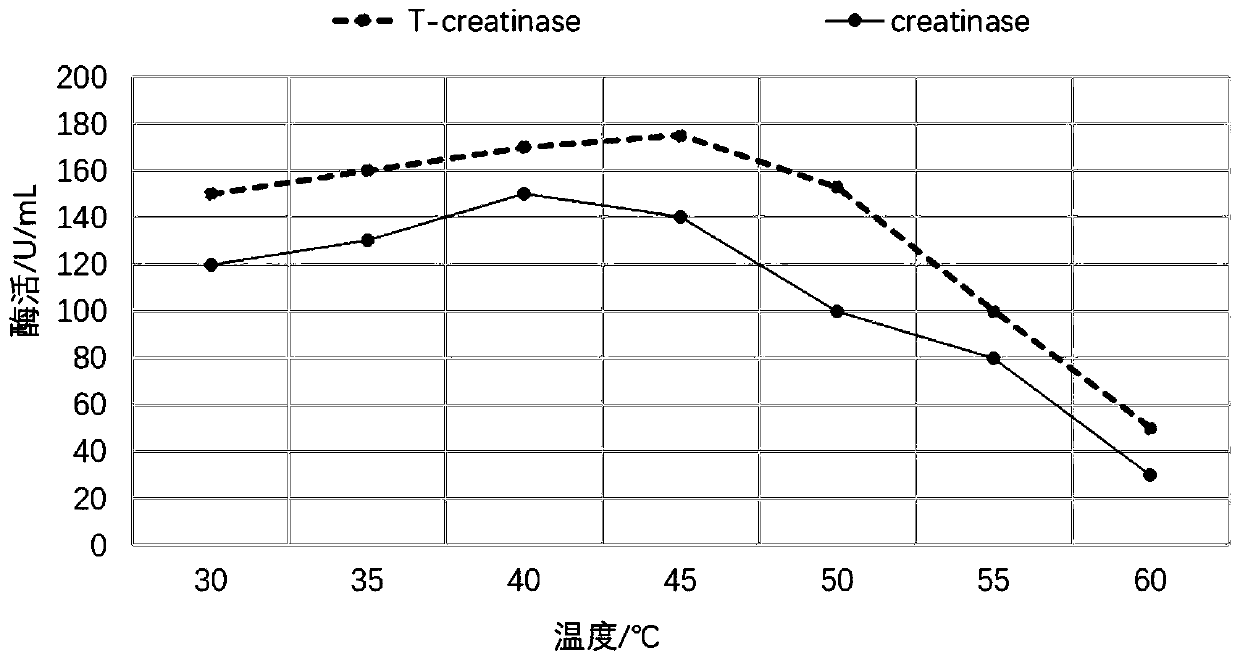
[0032] Based on the sequence of SEQ ID NO: 1 (from the patent CN104109658B), a mutation library was established by error-prone PCR, and the mutated target protein SEQ ID NO was obtained through gene screening and enzyme expression thermal stability and enzyme activity screening : Optimized gene for 2T-creatinase and SEQ ID NO:3. SEQ ID NO: 1: creatinase nucleotide sequence
[0033]
[0034]
[0035]
[0036] SEQ ID NO: 2T-creatinase amino acid sequence
[0037]
[0038] SEQ ID NO: 3 T-creatinase nucleotide sequence
[0039]
Embodiment 2
[0040] Embodiment 2: Construction of the expression system of mutant creatine hydrolase gene
[0041]The expression vector was selected as pET20b(+), the upstream restriction site was selected as NdeI, and the downstream restriction site was XhoI according to the carrier. The obtained cloning vector pMD19-T containing the T-creatinase of SEQ ID NO: 3 and the expression vector pET20b(+) were subjected to double enzyme digestion respectively. After digestion at 37° C. for 60 min, gel electrophoresis verified and the digestion products were recovered. The recovered expression vector was connected with the target gene, and the connection solution was transformed into the expression host E.coli-BL21, and the recombinant clone was verified to be correct by PCR.
Embodiment 3
[0042] Example 3: Heterologous expression of T-creatinase in recombinant Escherichia coli
[0043] Glycerol tube: the concentration of glycerol is 20%; the composition of the seed medium is (g / L): peptone 10, yeast extract 5, NaCl10, pH7.0, 100 μg / mL ampicillin; the composition of the fermentation medium is (g / L) : Peptone 12, yeast extract 24, glycerin 4, KH 2 PO 4 2.31,K 2 HPO 4 16.43, 100 μg / mL ampicillin. Seed culture: transfer 0.4% inoculum amount from glycerol tubes into 20mL / 250mL Erlenmeyer flasks, rotate on a rotary shaker at 200rpm, cultivate at 37°C for 12 hours. Fermentation culture: Inoculate the cultivated seed culture solution into a 50mL / 500mL Erlenmeyer flask at an inoculation amount of 1% (v / v) for cultivation, the cultivation temperature is 37°C, and the shaker speed is 200rpm. When OD600=0.6, add IPTG with a final concentration of 0.6mmol / L to induce expression, cultivate for 10h, the creatine hydrolase production can reach 125.6U / mL, and the control u...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com