Fluorescent probe DCCO and preparation method and application thereof
A technology of fluorescent probes and stock solutions, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of complex operation, low sensitivity, and inability to apply HClO/ClO- detection, etc., to achieve good selectivity, The effect of high sensitivity and short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 fluorescent probe DCCO
[0029] (1) 3-acetyl-7-(diethylamino)-2H-pyran-2-one (0.777g, 3mmol), 9-ethyl-9H-carbazole-3-formaldehyde (0.669g, 3mmol), dissolved in 15mL of ethanol / acetonitrile (1 / 1, V / V) mixed solution, piperidine (0.75mL, 7.5mmol) was added dropwise, heated to reflux for 30 hours, the reaction solution turned from orange to red, TLC Tracking Response (V 乙酸乙酯 :V 石油醚 =1:3);
[0030] (2) After the reaction is over, the solvent is removed under reduced pressure, and the gradient elution method is used for column chromatography separation, first eluted with ethyl acetate / petroleum ether (1 / 3, V / V), and then ethyl acetate / petroleum ether (1 / 2, V / V) elutes, obtains 0.699g crude product;
[0031] (3) The crude product was dissolved in ether, filtered, and dried to obtain 0.349 g of an orange-yellow pure product with a yield of 25%.
[0032] For fluorescent probes 1 H NMR characterization, the results are as follows:
[0033] ...
Embodiment 2
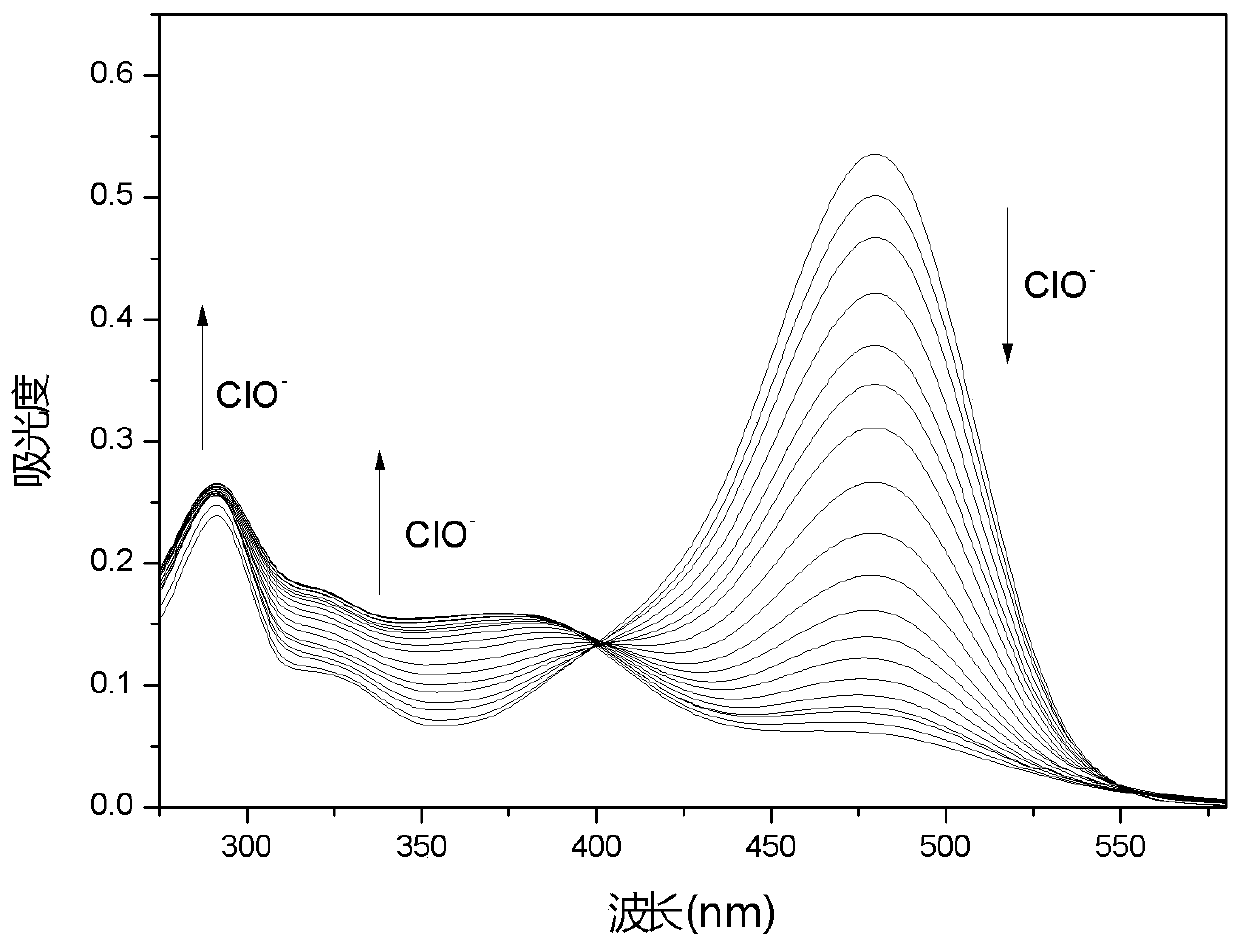
[0035] Embodiment 2 fluorescent probe DCCO with ClO - Varying UV Absorption Spectrum
[0036] Add 10.0 μL of fluorescent probe stock solution to 2.0 mL of water / DMSO (1 / 1, v / v) system for ClO - UV titration experiment, and record its UV absorption spectrum ( figure 1 ). With ClO - With the increase of the amount, the ultraviolet absorption at 290nm, 321nm and 355nm all increased, and the ultraviolet absorption at 480nm decreased.
Embodiment 3
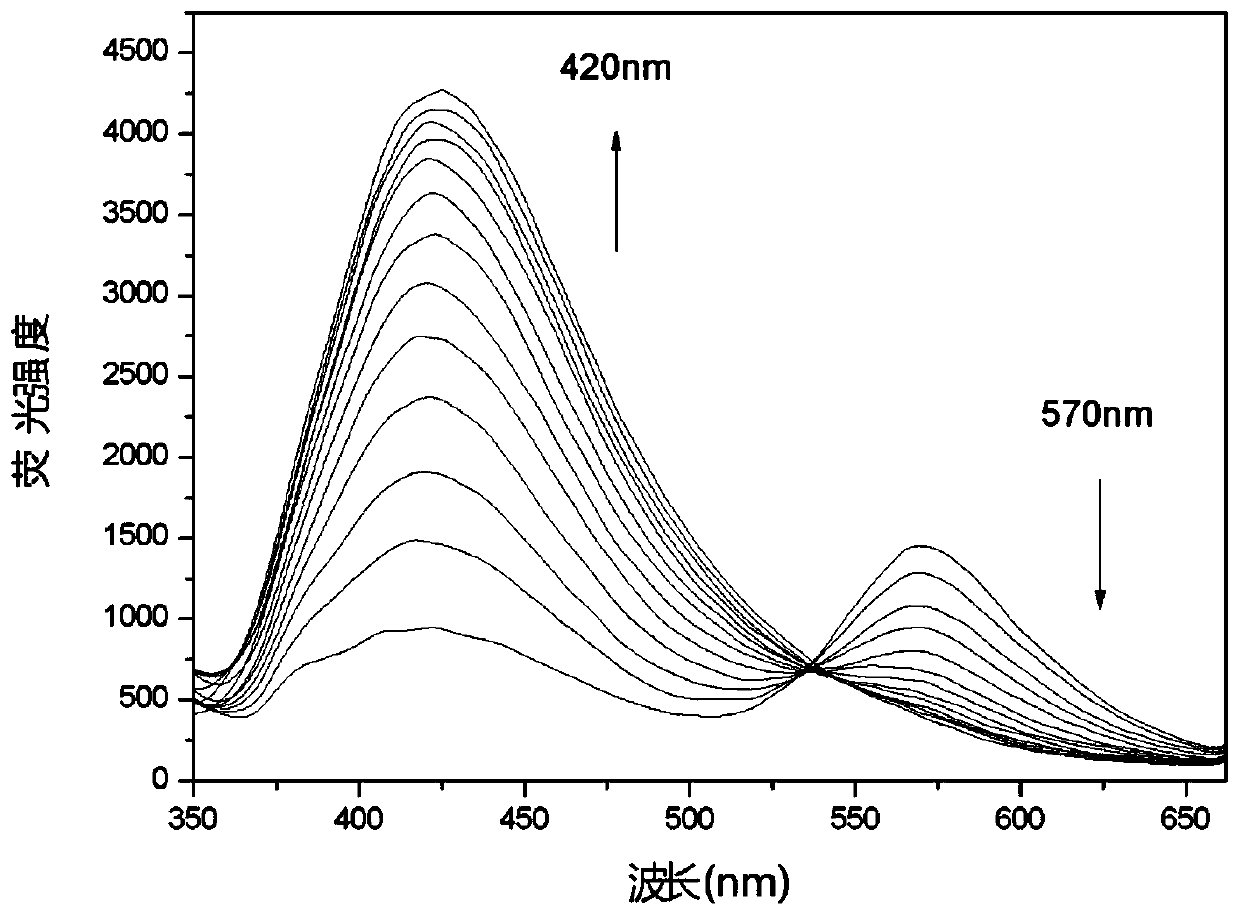
[0037] Embodiment 3 fluorescent probe DCCO with ClO - Fluorescence titration plot of change
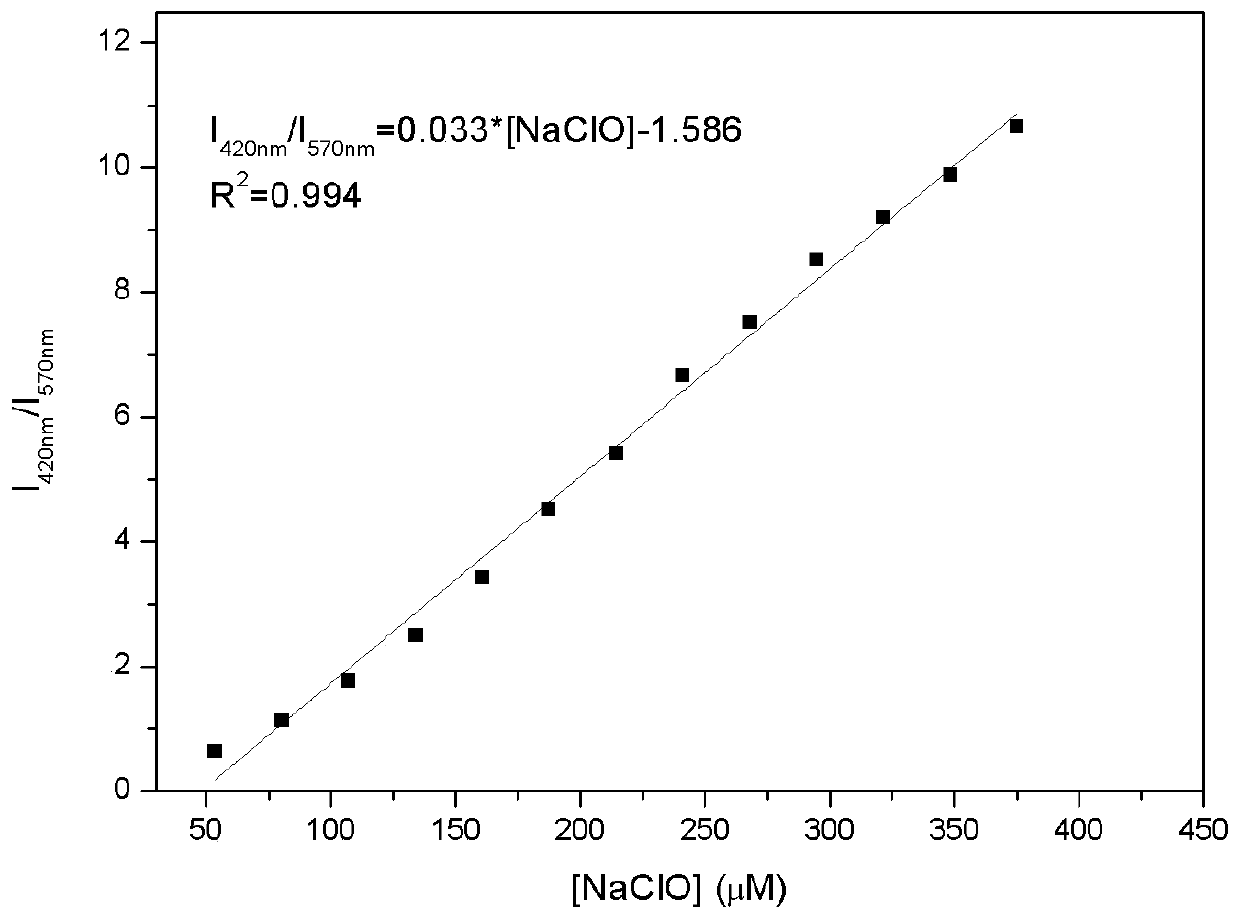
[0038] Add 10.0 μL fluorescent probe stock solution to 2.0 mL water / DMSO (1 / 1, v / v) system for ClO -Fluorescence titration experiment, detected on a spectrofluorometer, with ClO - With the increase of , the fluorescence intensity at 570nm gradually weakened, and a new peak appeared at 420nm and gradually increased ( figure 2 ). Instrument parameters: the slit widths of the excitation wavelength and emission wavelength are 5.0nm and 5.0nm respectively, the voltage is 600V, and the maximum excitation wavelength of the fluorescent probe solution is: λ ex 335nm and a maximum emission wavelength of λ em 570nm. Fluorescence ratio I 420nm / I 570nm Plotting the graph for the ordinate gives ClO - Concentration working curve, the linear regression equation is I 420nm / I 570nm =0.033*[NaClO]-1.586, the unit of NaClO concentration is 10 -6 mol / L; the linear correlation coefficient is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com