Porous PCL-TCP (polycaprolactone-tricalcium phosphate) artificial bone scaffold with drug sustained release function and preparation method of porous PCL-TCP artificial bone scaffold
An artificial bone and drug technology, applied in the fields of medical formula, drug delivery, medical science, etc., can solve the problems of reducing osteoblast activity and bone reconstruction function, cortical bone microporous bone strength, and bone defect difficult to heal, etc., to achieve Good mechanical support and degradable properties, promoting early osseointegration and osseointegration, and good osseointegration ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
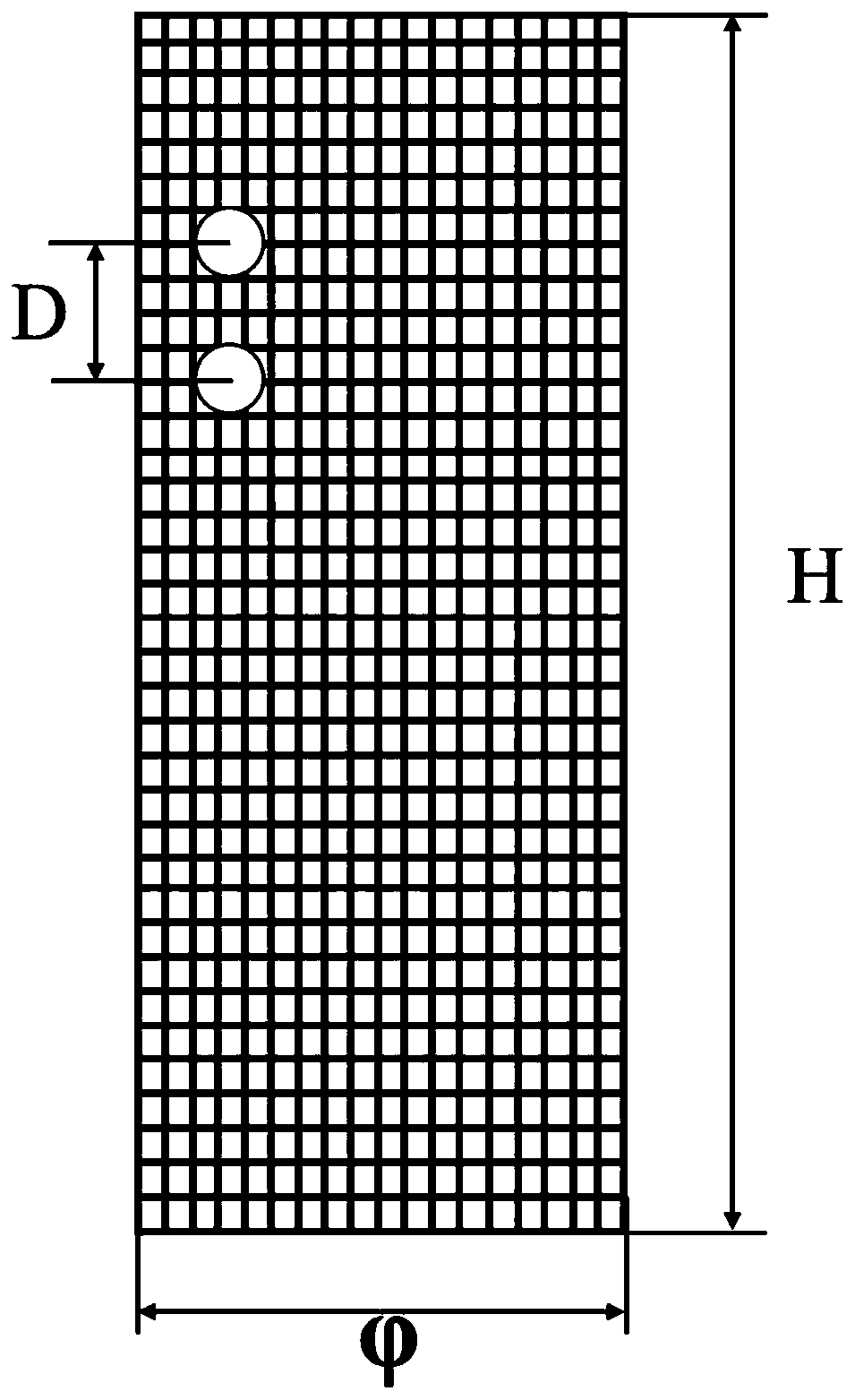
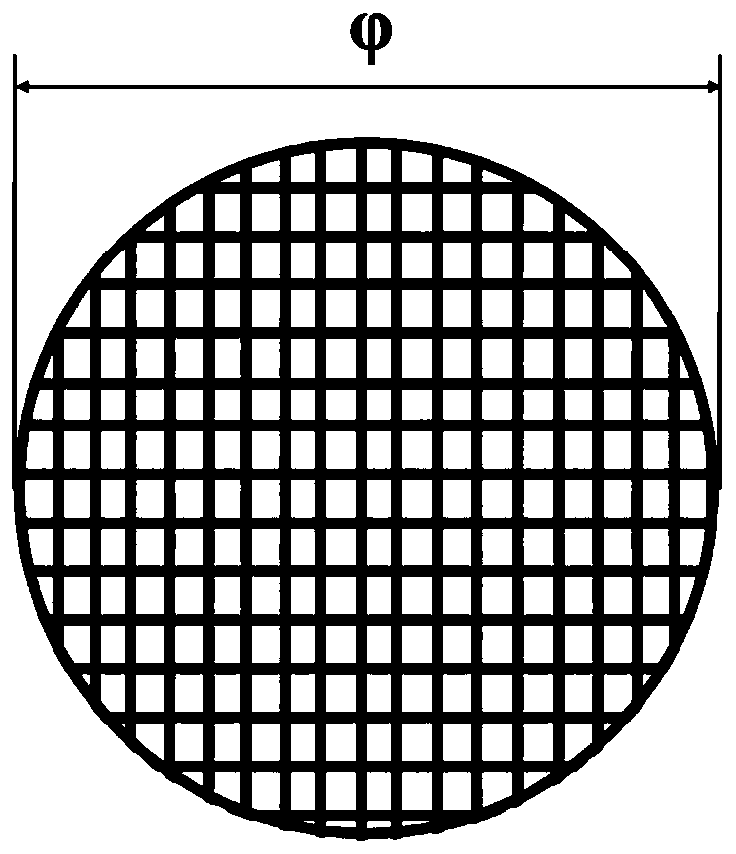
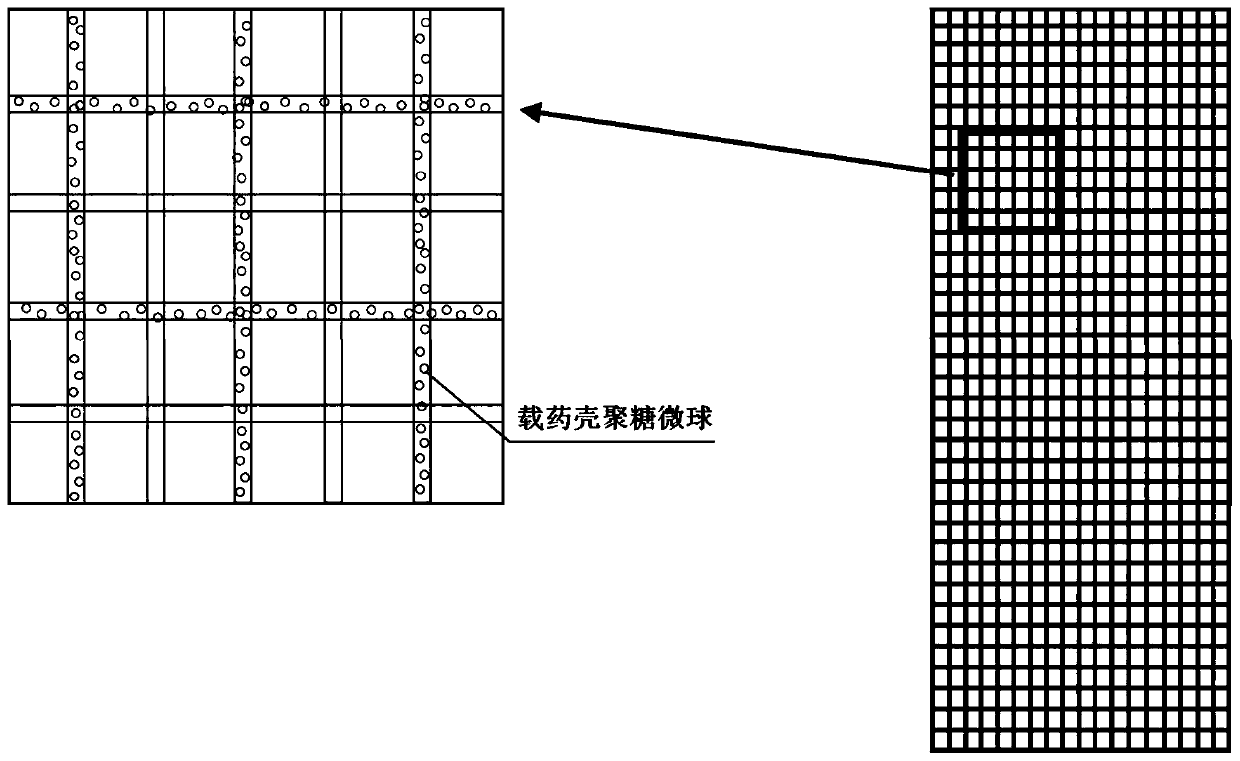
[0025] The present invention will be described in further detail below in conjunction with the accompanying drawings.
[0026] 1) Preparation of chitosan microspheres loaded with bone formation-promoting drugs by emulsification cross-linking method
[0027] First, take 100mg ~ 1500mg chitosan and dissolve it in 8 ~ 75ml dilute acetic acid solution with a volume concentration of 1 ~ 5%, and ultrasonically disperse the chitosan to completely dissolve the chitosan solution; secondly, according to 1:1 ~ 8: Add bone-promoting drugs (parathyroid hormone, fluoride, growth hormone or statins) to a zinc acetate solution with a volume concentration of 1% to 10%, incubate at 4°C for 24 to 48 hours, freeze Dry collection of Zn-drug powder;
[0028] Then, stir the chitosan solution and the Zn-drug powder evenly according to the volume-to-mass ratio of 5:1-30:1, and slowly add 50ml-300ml of Span 80 liquid paraffin solution with a volume concentration of 1%-5%. , stir evenly, and then drop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Compressive strength | aaaaa | aaaaa |
| Compressive modulus | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com