A kind of carbon dioxide electrochemical reduction catalyst and preparation method thereof
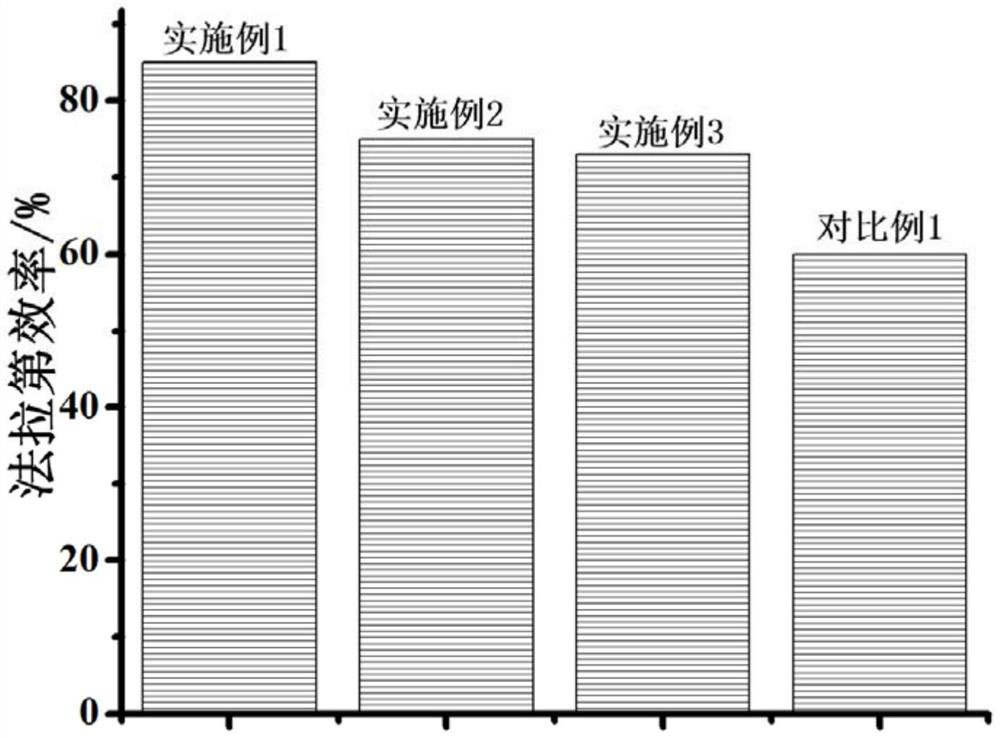
A carbon dioxide and catalyst technology, applied in the field of carbon dioxide electrochemical reduction catalyst and its preparation, can solve the problems of poor catalyst stability, low current efficiency, and low selectivity of target products, achieve high electrocatalytic activity and selectivity, and increase contact ratio Surface area, effect of increasing Faradaic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation method of the carbon dioxide electrochemical reduction catalyst is characterized in that it comprises the following steps:
[0020] (1) Synthesis of polystyrene microspheres: First, add 80 mL of styrene monomer and 8 mL of acrylic acid into 400 mL of distilled water, stir and disperse, then use a water bath to heat the temperature of the reaction system to 60°C; then pass nitrogen gas for 30 minutes to remove the Then add 15mL of potassium persulfate aqueous solution with a concentration of 40mmol / L, and continue to react for 18h under the stirring condition of 250r / min to obtain an emulsion; after the obtained emulsion is centrifuged, the centrifuged product is washed with ethanol for 3 times, and then vacuum freeze-dried Freeze-dry the polystyrene microspheres under the condition of 20Pa, -40℃, and set aside;
[0021] (2) Synthesis of copper oxide hollow microspheres: take 2 g of copper sulfate, 0.3 g of sodium hydroxide and 1.5 g of polystyrene micros...
Embodiment 2
[0024] The preparation method of the carbon dioxide electrochemical reduction catalyst is characterized in that it comprises the following steps:
[0025] (1) Synthesis of polystyrene microspheres: First, add 50 mL of styrene monomer and 5 mL of acrylic acid into 300 mL of distilled water, stir and disperse, then use a water bath to heat the temperature of the reaction system to 50°C; then pass nitrogen gas for 20 minutes to remove the Then add 10mL of potassium persulfate aqueous solution with a concentration of 30mmol / L, and continue to react for 10h under the stirring condition of 200r / min to obtain an emulsion; after centrifuging the obtained emulsion, wash the centrifuged product with ethanol for 3 times, and then use vacuum freeze-drying Freeze-dry the polystyrene microspheres under the condition of 20Pa, -40℃, and set aside;
[0026] (2) Synthesis of copper oxide hollow microspheres: take 1g of copper sulfate, 0.1g of sodium hydroxide and 1g of polystyrene microspheres ...
Embodiment 3
[0029] The preparation method of the carbon dioxide electrochemical reduction catalyst is characterized in that it comprises the following steps:
[0030] (1) Synthesis of polystyrene microspheres: First, add 100 mL of styrene monomer and 10 mL of acrylic acid into 500 mL of distilled water, stir and disperse, then use a water bath to heat the temperature of the reaction system to 70°C; then pass nitrogen gas for 40 minutes to remove the Then add 25mL of potassium persulfate aqueous solution with a concentration of 50mmol / L, and continue to react for 24h under the stirring condition of 300r / min to obtain an emulsion; after the obtained emulsion is centrifuged, the centrifuged product is washed with ethanol for 3 times, and then vacuum freeze-dried Freeze-dry the polystyrene microspheres under the condition of 20Pa, -40℃, and set aside;
[0031] (2) Synthesis of copper oxide hollow microspheres: take 3 g of copper sulfate, 0.5 g of sodium hydroxide and 2 g of polystyrene micros...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com